HOVON HO87 MM
Main info
- Identifier:
- HO87 MM
- Sponsor:
- HOVON
- Working group party:
- Myeloma
- Age:
- > 65
- Stage:
- 1st Line
- Echelon:
- Level D
- Included patients:
-
668(of 668)
- Active sites:
-
107(of 49)
- Title:
Randomized phase III trial in elderly patients with previously untreated symptomatic Multiple Myeloma comparing MP-Thalidomide (MP-Thal) followed by thalidomide maintenance versus MP-Lenalidomide (MP-Len) followed by maintenance with lenalidomide.
Timeline
Flow
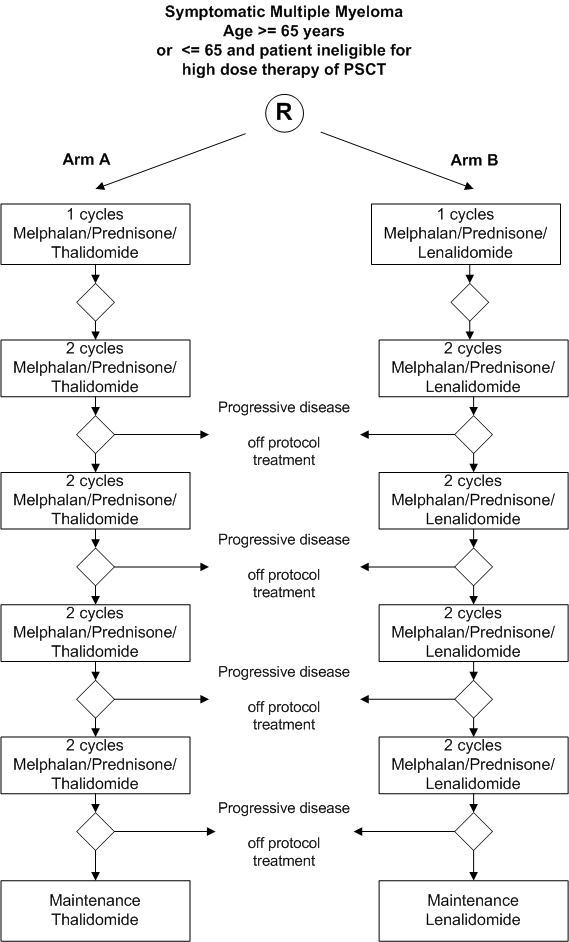
Details
- Phase:
- Prospective Phase III study
- Monitoring Type:
- Not any more
- Objectives:
Primary endpoint
- Progression free survival, defined as time from registration to progression or death from any cause
- Response rate defined as sCR, CR or VGPR
Secondary endpoints
- Overall response rate defined as sCR, CR, VGPR or PR
- Overall survival, measured from time of registration
- Quality of response during maintenance, measured as improvement of response (from start maintenance till progression)
- Time to maximum response, defined as time from registration to maximum response
- Time to death from relapse/progression (after initial response), measured from time of first relapse/progression
- Safety and toxicity as defined by type, frequency and severity of adverse events as defined by the National Cancer Institute (NCI) Common Terminology Criteria (CTC), version 3.0
- Quality of life as defined by the EORTC QLQ-C30 definitions.
Eligibility
- Inclusion Criteria:
- Previously untreated patients with a confirmed diagnosis of symptomatic multiple myeloma according to IMWG criteria (see appendix A)
- Age > 65 years or patients ≤ 65 not eligible for high dose chemotherapy and peripheral stem cell transplantation
- WHO performance status 0-3 for patients <75 years and WHO performance status 0-2 for patients ≥ 75 years (see appendix E)
- Measurable disease as defined by the presence of M-protein in serum or urine or proven plasmacytoma by biopsy (see appendix A for definitions)
- Written informed consent
- Exclusion Criteria:
- Non-secretory MM
- Known hypersensitivity to thalidomide
- Systemic AL amyloidosis
- Polyneuropathy, grade 2 or higher
- Severe cardiac dysfunction (NYHA classification II-IV, appendix F)
- Severe pulmonary dysfunction
- Significant hepatic dysfunction (total bilirubin ≥ 30 μmol/l or transaminases ≥ 3 times normal level), unless related to myeloma
- Creatinine clearance <30 ml/min
- Patients with active, uncontrolled infections
- Pre-treatment with cytostatic drug, IMIDs or proteasome inhibitors. Radiotherapy or a short course of steroids (e.g. 4 day treatment of dexamethasone 40 mg/day or equivalent) are allowed.
- Patients known to be HIV-positive
- History of active malignancy during the past 5 years, except basal carcinoma of the skin or stage 0 cervical carcinoma
- Not able and/or not willing to use adequate contraception
- Pregnancy
Registration Details
Eligible patients should be randomized before start of treatment. Patients need to be registered at the HOVON Data Center of the Erasmus MC Rotterdam via the Internet via TOP (Trial Online Process; https://www.hdc.hovon.nl/top) or by phone call: +31.10.7041560 or fax +31.10.7041028 Monday through Friday, from 09:00 to 17:00 CET. A logon to TOP can be requested at the
HOVON Data Center for participants.
The following information will be requested at registration:
- Protocol number
- Institution name
- Name of caller/responsible investigator
- Sex
- Date of birth
- Date written informed consent
- ISS stage
- Will patient participate in the quality of life study
- ‘Risk Management Program’ discussed with patient
- Approval for blood storage for scientific research
- Approval for bone marrow storage for scientific research
- Eligibility criteria
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
