------ HO90 NHL
Main info
- Identifier:
- HO90 NHL
- Sponsor:
- ------
- Working group party:
- Lymphoma
- Age:
- 18-60
- Stage:
- 1st Line
- Included patients:
-
136
- Active sites:
-
30(of 29)
- Title:
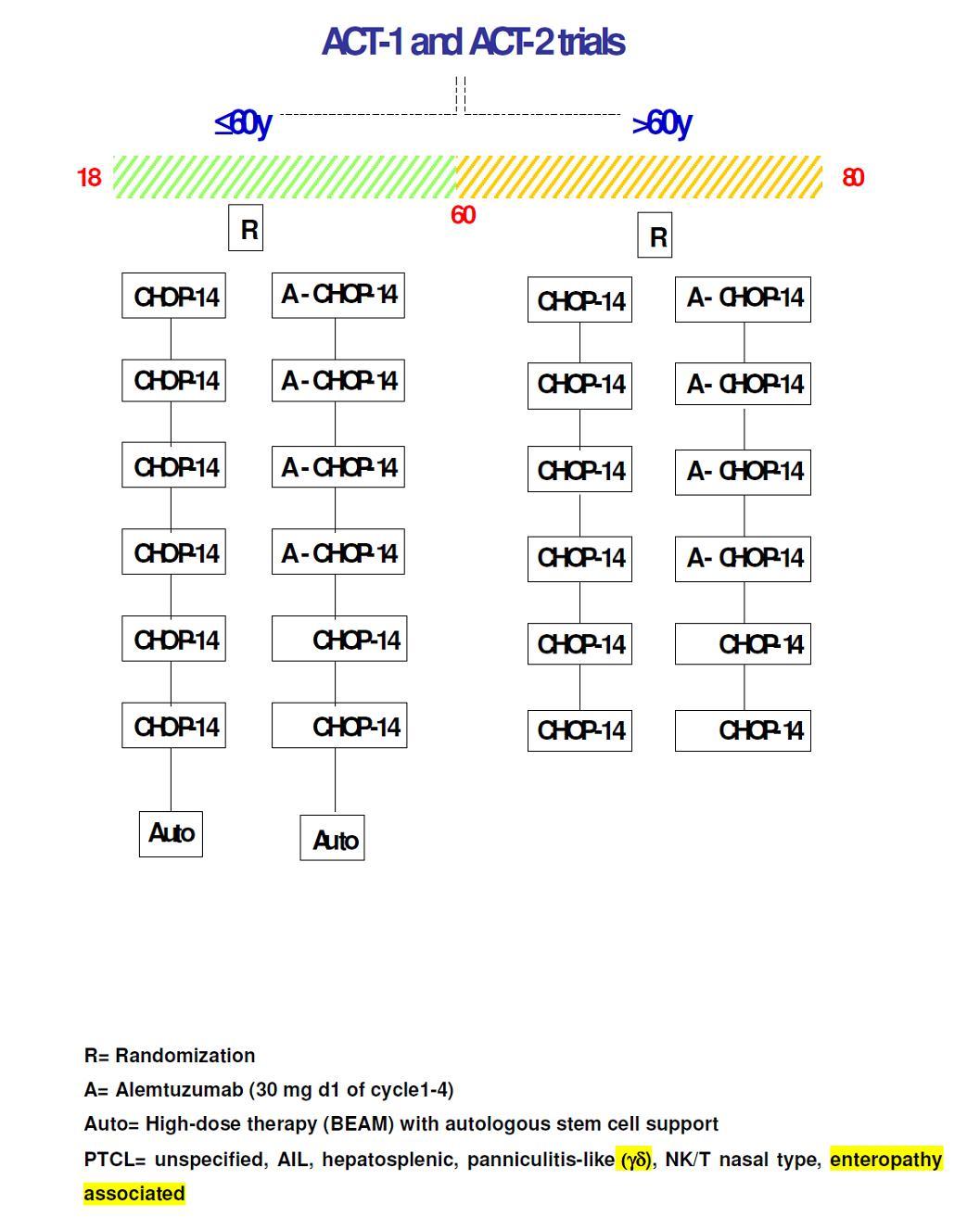
ACT-1 (younger patients) A randomized phase III study to evaluate the efficacy of chemoimmunotherapy with
the monoclonal antibody Campath-1H (Alemtuzumab) given in combination with 2-weekly CHOP versus 2-weekly CHOP alone and consolidated by autologous stem cell transplant, in young patients with previously untreated systemic peripheral Tcell lymphomas
Timeline
News
- Both amendment 1 & 2 have been approved for the HO90 T-NHL (ACT1) study.
- After receipt of the protocol signature pages, adjusted ICF and signed checklist at the HOVON Data Center, patients can be included again.
- The HO90 T-NHL study has been initial approved by the METC of the UMC Groningen on 25-NOV-2008.
- This study is a so-called intergroup study in which HOVON participates. Leading group is the Nordic Lymphoma Group (Prof F. d'Amore). If you wish to participate in this study please contact the HOVON Data Center (M. Luten), before taking any action.
- Please notice that a FAQ document is available for this study. If you have any other question regarding this study, do not hesitate to contact the HOVON Data Center.
Flow
Details
- Phase:
- Prospective randomized Phase III study
- Monitoring Type:
- Not any more
- Objectives:
Primary objective:
To assess the effect of the addition of alemtuzumab s.c. to 6 courses of 2-weekly CHOP14 in terms of Event-Free Survival (EFS) as defined in paragraph 16.1.Secondary Objectives:
- To assess the effect of the addition of alemtuzumab s.c. to 6 courses of 2-weekly CHOP14 in term of overall survival (OS), progression-free survival (PFS) and overall response rates (ORR, i.e. CR/CRu/PR), tumor control or time to progression (TTP).
Assessment of ORR means evaluating eligibility to autologous stem cell transplantation.
- To assess the overall response rates (ORR) related to the CD52 expression.
- To evaluate the safety of the addition of alemtuzumab s.c. combined with 2-weekly CHOP with respect to the incidence of severe opportunistic infections and infections due to neutropenia as well as the adherence to protocol as defined in paragraph 16.2.
Eligibility
- Inclusion Criteria:
- Previously untreated patients with newly diagnosed peripheral T-cell lymphoma of stage I bulk ( ≥ 7.5 cm) and stages II to IV.
- Patients with a confirmed histologic diagnosis of peripheral T-cell NHL according to the WHO classification (Appendix C):
- Peripheral T-cell lymphoma, unspecified (PTCL NOS)
- Angioimmunoblastic T-cell lymphoma
- Enteropathy associated T cell lymphoma
- Subcutaneous panniculitis-like T-NHL (gd T-cell lymphoma)
- Hepatosplenic T-cell lymphoma
- Extranodal NK/T cell lymphoma, nasal type
- Age 18-60 years at time of randomization
- Life expectancy of 3 months or longer
- ECOG performance status (PS) 0, 1 or 2 at the time of randomization (see appendix D). However, PS 3 will be acceptable if lymphoma-related.
- Measurable disease (defined as at least one lesion with two measurable perpendicular diameters of which at least one should be ≥ 15 mm).
- Written informed consent
- Exclusion Criteria:
- Patients with NK/T-NHL of the following type:
- Precursor T cell lymphoblastic lymphoma/leukemia
- All mature T cell leukemias (T-PLL, ATLL, NK cell leukemia, T-LGL, HTLV1-pos ATL)
- Alk-positive and negative anaplastic large cell lymphoma
- Blastic NK cell lymphoma
- Cutaneous T-cell lymphoma, transformed or not
- Concurrent severe and/or uncontrolled medical disease (e.g. uncontrolled diabetes, congestive heart failure, myocardial infarction within 6 months prior to the study, unstable and uncontrolled hypertension, chronic renal disease, or active uncontrolled infection), which could compromise participation in the study.
- Known hypersensitivity to murine or chimeric antibodies or proteins
- Severe cardiac dysfunction (NYHA classification II-IV, Appendix H) or LVEF < 45 %
- Significant renal dysfunction, i.e. serum creatinin >2 times upper normal level (UNL), unless related to NHL
- Significant hepatic dysfunction (total bilirubin >2 times UNL or transaminases ≥ 2.5 times UNL), unless related to NHL
- Impaired pulmonary functions; in this case, the patient is to be excluded if the resultant pulmonary function test shows FEV1<50% or a diffusion capacity <50% of the reference values
- Suspected or documented Central Nervous System involvement by NHL
- Patients known to be HIV-positive
- Patients with active, uncontrolled infections, especially known seropositivity for HCV or HbsAg
- Patients with uncontrolled asthma or allergy, requiring systemic steroid treatment
- Prior treatment with chemotherapy, radiotherapy or immunotherapy for this lymphoma, except local radiotherapy in case of extranodal NK/T cell lymphoma, nasal or nasal type
- History of active cancer during the past 5 years, except basal carcinoma of the skin or stage 0 cervical carcinoma
- Unwillingness or inability to comply with the protocol
- Simultaneous participation in any other study protocol
- Pregnant and nursing women (Women of childbearing potential should use safe anticonception (contraceptive pills, intrauterine devices, injection of prolonged gestagen, subdermal implantation, hormonal vaginal devices and transdermal patches are
considered as safe contraceptive methods).
- Patients with NK/T-NHL of the following type:
Registration Details
The patient should be registered and randomized immediately after satisfactory completion of screening tests and obtaining informed consent, and before the start of chemotherapy. Patients need to be registered at the Clinical Trial Office.
The following information will be requested at randomization:
- Protocol number
- Institution name
- Name of caller/responsible investigator
- Patient’s initials or code
- Patient’s hospital record number
- Sex
- Date of birth
- Date of diagnosis of NHL
- WHO classification
- Pathology result from referral/reference pathologist
- Eligibility criteria (i.e. all inclusion and exclusion criteria)
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
