HOVON HO900 NHL
Main info
- Identifier:
- HO900 NHL
- Sponsor:
- HOVON
- Working group party:
- Lymphoma
- Age:
- >= 18
- Echelon:
- Level D
- Included patients:
-
1200(of 1200)
- Active sites:
-
64(of 64)
- Title:
A national MYC screening study for newly diagnosed DLBCL patients.
Timeline
News
The HOVON 900 DLBCL study is now closed for inclusion.
Flow
Details
- Phase:
- Select:
- Monitoring Type:
- Objectives:
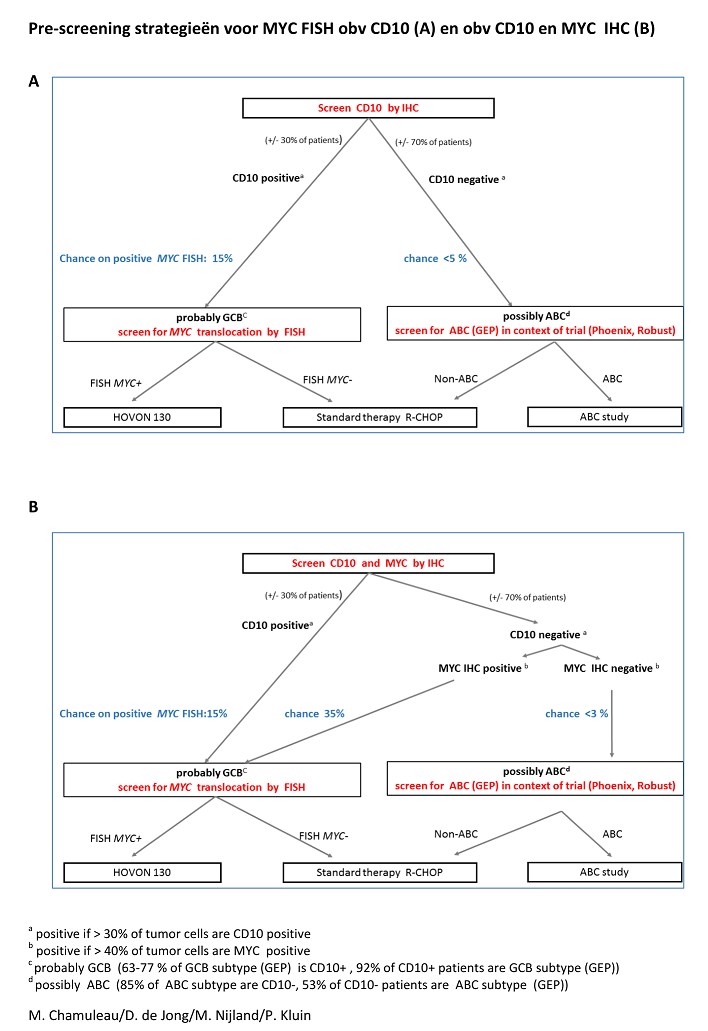
The primary objective is to effectively identify patients who have a MYC rearrangement, to collect anonymized data of all DLBCL patients (MYC positive and negative) for epidemiological studies and to install a virtual tissue bank for future comparative MYC-related biological research.
Eligibility
- Inclusion Criteria:
- All patients diagnosed with newly diagnosed DLBCL according to WHO 2016. (patients with transformed follicular lymphoma are not eligible)
- Age ≥ 18 years
- Within 8 weeks after the diagnosis of DLBCL
- Exclusion Criteria:
NA
Registration Details
Eligible patients should be registered before start of treatment. Patients need to be registered at the HOVON Data Center by one of the following options:
- By ALEA; Use goto eCRF button > select the [Patient tab] and click the [Add new patient] button. Complete all items and click the [Submit] button
- By faxing the completed registration/randomization CRF +31 (0)10 704 1028 Monday through Friday, from 09:00 to 17:00 CET
- By phone +31 (0)10 704 1560 Monday through Friday, from 09:00 to 17:00 CET
The following information will be requested at registration:
- Protocol number
- Institution name
- Name of caller/responsible investigator
- Local patient code (optional)
- Sex
- Date of birth (or partial date of birth if date of birth not allowed, e.g.01/01/YOB)
- Date written informed consent
- Specific items for which this patient gives consent (see ICF)
- Date of diagnosis
- Diagnosis (DLBCL)
- Name of the local pathology laboratory
- Local pathology registration number
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
