EBMT HO93 AML
Main info
- Identifier:
- HO93 AML
- Sponsor:
- EBMT
- Working group party:
- SCT & Supportive care
- Age:
- >= 60 - <= 75
- Stage:
- 1st Line
- Echelon:
- Level A
- Included patients:
-
33(of 33)
- Active sites:
-
6(of 5)
- Title:
A Randomized Phase III study comparing conventional chemotherapy to low dose
total body irradiation-based conditioning and hematopoietic cell transplantation from related and unrelated donors as consolidation therapy for older Patients with AML in first Complete Remission HCT vs CT in elderly AML
Timeline
News
As per Thursday, 20 October 2016 the HOVON-93 study will be closed for inclusion of new patients for Dutch Centers. It was decided to close the study prematurely because of the low inclusion.
Please inform your local staff that patients, who have signed already informed consent, can still be included in the study.
Please contact Marlies Groenendijk when you have a patient that have already signed the informed consents.
We thank you for your good cooperation.
Yours sincerely,
Marlies Groenendijk (trialmanager)
Flow
Details
- Phase:
- Prospective Phase III study
- Monitoring Type:
- Not any more
- Objectives:
Primary objective:
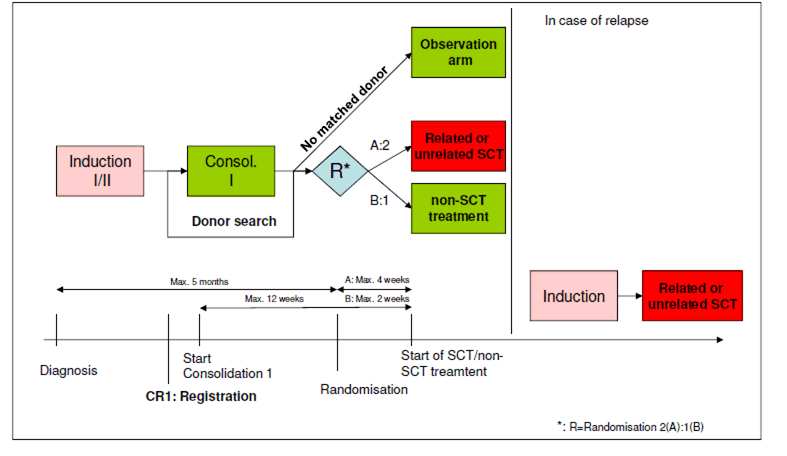
To evaluate leukaemia free survival (LFS) for older patients after allogeneic SCT in AML/RAEB in CR using matched or unrelated donors in comparison to conventional chemotherapy.Secondary objectives:
To evaluate:- E8Overall Survival
- Cumulative incidence of relapse
- TRM and complications
- Incidence of myelosuppression (ANC < 500/mm³ for > 2 days, platelets < 20,000/mm³ for > 2 days) after initial PBSC infusion
- Incidence of grades 2-4 acute GvHD after transplant
- Incidence of grades chronic extensive GvHD after DLI
Eligibility
- Inclusion Criteria:
Inlcusion criteria at registration:
- Age ≥ 60 years and ≤ 75 years
- primary or secondary AML as defined by WHO or refractory anemia with excess of blasts (RAEB)
- First complete remission following one or two cycles of induction chemotherapy
- Chemotherapy was administered according to current participating cooperative group protocols
- Karnofsky score > 70 (see Appendix D. - Karnofsky performance scale)
- Written informed consent
Inclusion criteria at randomisation:
- Patient is registered in this trial
- Complete remission must be confirmed after first consolidation cycle according to the response criteria enlisted in appendix B with the following exception regarding the peripheral blood recovery (B1):
- Peripheral Blood Recovery (PBR): ANC ≥ 1.0 x 10^9/l or 1500/mm³, transfusion independent platelet count ≥ 50 x 10^9/l (i.e. 48 h after last transfusion) and no leukemic blasts in the peripheral blood and no dysplasia
- AND platelet count must be increasing
- Matching (10/10: HLA-A, -B, -C, DRB1 and DQ) related or unrelated donor is available
- Exclusion Criteria:
Exclusion criteria at registration:
- AML FAB M3
- HIV positivity
- Participation in another clinical trial without prior consent of the coordinating investigator, patients may exceptionally take part in a further study only if
- The second study exclusively concerns induction therapy
- Consolidation cycle one and two are given according to the accredited study group policy
- No investigational drugs are used post registration for the HCT vs CT in eldery AML study.
- Documentation for the HCT vs CT in eldery AML study is not compromised. Second hand data from foreign study is not accepted
Exclusion criteria at randomisation:
- Patient has undergone more than one consolidation cycle
- More than 5 months (>150 days) after diagnosis
- Organ dysfunction
- Patients with creatinine clearance < 50 ml/min
- Cardiac ejection fraction < 40%
- Severe defects in pulmonary function testing (defects are currently categorized as mild, moderate and severe) as defined by the pulmonary consultant, or receiving supplementary continuous oxygen
- Liver function tests: total bilirubin > 2x the upper limit of normal, SGOT and SGPT 4x the upper limit of normal
- Patients with poorly controlled hypertension
* Participation in another clinical trial without prior consent of the coordinating investigator
Exclusion criteria donor:
- Participation in another clinical trial without prior consent of the coordinating investigator
- Monozygotic identical twin
- Age ≤ 18 years
- Pregnancy
- Infection with HIV
- Inability to achieve adequate venous access
- Known allergy to G-CSF
- Current serious systemic illness
Registration Details
All study centres will register the eligible patients via the completed and signed CRF registration form to the ZKS Leipzig – KKS (KKS) Data Management as per contact details on the form. The KKS will send a confirmation of the registration if all registration
criteria are met.
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
