HOVON HO95 MM
Main info
- Identifier:
- HOVON 95 MM / EMN02
- Sponsor:
- HOVON
- Working group party:
- Myeloma
- Age:
- 18-65
- Stage:
- 1st Line
- Echelon:
- Level D
- Included patients:
-
1503(of 1500)
- Active sites:
-
182(of 189)
- Title:
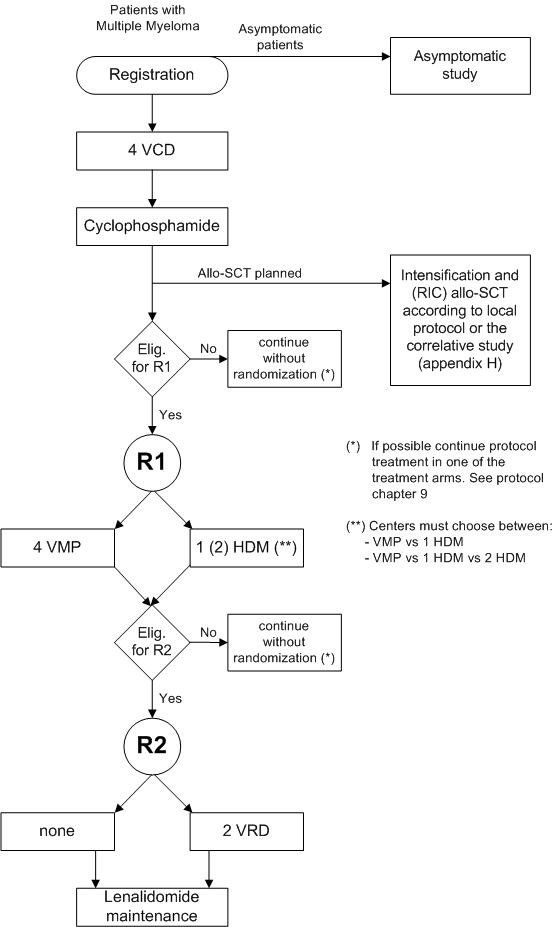
A randomized phase III study to compare Bortezomib, Melphalan, Prednisone (VMP) with High Dose Melphalan followed by Bortezomib, Lenalidomide, Dexamethasone (VRD) consolidation and Lenalidomide maintenance in patients with newly diagnosed multiple myeloma.
Timeline
News
- AM9 (protocol version 8 dd 29MAR2021). Maintenance treatment with study IMP will be given till 10 years after inclusion. Patients still on maintenance treatment will proceed with maintenance at the discretion of the physician outside the scope of the study.
- For data management go to the EMN website: (http://www.mm-sen.net/)
- Sampling (at relapse/progression to central lab) IMPORTANT: it is not possible to ship material on a Friday, Saturday or Sunday. If your hospital routinely has multiple myeloma patient appointments for obtaining clinical samples on these days, please inform the PI and please change the planning. Seeing patients for MM biomaterial sampling on a Friday or on the weekend is in direct conflict with participating in a clinical trial such as this one.
- Updated documents:
08DEC21: New documents regarding AM 09
- Protocol
- Protocol SoC; Tracked Changes
- Signature page of protocol
- Patient Addendum Information & AOR form (NL)
- Patient letter-change independent physician (NL)
- Patient Addendum Information & AOR form (co-sponsor)
- Patient Addendum Information & IC form / data processing information (co-sponsor)
- CCMO response (NL)
- MEC approval (NL)
- Signature page of protocol
Flow
Details
- Phase:
- Prospective randomized Phase III study
- Monitoring Type:
- Not any more
- Objectives:
Primary
- Efficacy of FCM in correction of ID (TSAT >=20%)
- Impact of FCM on quality of life (EORTC QLQ-C30)
Secondary
Impact of FCM on:- Haemoglobin levels during induction therapy
- Number of stem cells collected
- Transfusion rate during induction
- Transfusion rate after stem cell transplantation (<21 days)
- Response to VCD induction chemotherapy
- Toxicity
Eligibility
- Inclusion Criteria:
- Patients with a confirmed diagnosis of symptomatic multiple myeloma stage I to III according to the International Staging System ISS (see appendix A), i.e. at least one of the CRAB criteria should be present;
- Measurable disease as defined by the presence of M-protein in serum or urine (serum Mproteïn > 10 g/l or urine M-proteïn > 200 mg/24 hours or abnormal FLC ratio with involved free light chain (FLC) > 100 mg/l) or proven plasmacytoma by biopsy;
- Age 18-65 years inclusive;
- WHO performance status 0-3 (WHO=3 is allowed only when caused by MM and not by comorbid conditions) (see appendix D);
- Negative pregnancy test at inclusion if applicable;
- Written informed consent.
Inclusion criteria for randomization 1:
- WHO performance 0-2;
- Bilirubin and transaminases < 2.5 times the upper limit of normal values;
- A suitable stem cell graft containing at least 4 x 10^6 CD34+ cells/kg (or according to national guidelines).
Inclusion criteria for randomization 2:
- Bilirubin and transaminases < 2.5 times the upper limit of normal values;
- ANC ≥ 0.5 x 10^9/l and platelets > 20 x 10^9/l;
- Patient is able to adhere to the requirements of the Lenalidomide Pregnancy Prevention Risk Management Plan.
- Exclusion Criteria:
- Known intolerance of Boron;
- Systemic AL amyloidosis;
- Primary Plasmacell Leukemia;
- Non-secretory MM;
- Previous chemotherapy or radiotherapy except local radiotherapy in case of local myeloma progression or corticosteroids maximum 5 days for symptom control;
- Severe cardiac dysfunction (NYHA classification II-IV, see appendix E);
- Significant hepatic dysfunction (serum bilirubin ≥ 30 μmol/l or transaminases ≥ 2.5 times normal level), unless related to myeloma;
- Patients with GFR <15 ml/min,
- Patients known to be HIV-positive;
- Patients with active, uncontrolled infections;
- Patients with neuropathy, CTC grade 2 or higher;
- Patients with a history of active malignancy during the past 5 years with the exception of basal carcinoma of the skin or stage 0 cervical carcinoma;
- Patients who are not willing or capable to use adequate contraception during the therapy (all men, all pre-menopausal women);
- Lactating women.
Exclusion criteria for randomization 1:
- Severe pulmonary, neurologic, or psychiatric disease;
- CTCAE grade 3-4 polyneuropathy during Bortezomib treatment;
- Allogeneic Stem Cell Transplantation (Allo SCT) planned;
- Progressive disease.
Exclusion criteria for randomization 2:
- Progressive disease;
- Neuropathy, except CTCAE grade 1;
- CTCAE grade 3-4 polyneuropathy during Bortezomib treatment.
Registration Details
The patient should be registered immediately after diagnosis and before the start of chemotherapy.
Patients will be registered at the EMN Data Center by web http://www.mm-sen.net. Investigators who do not have an account yet should register at this website to obtain an account.
The following information will be requested at registration:
- Institution name
- Name of responsible investigator
- Date of birth
- Date of informed consent
- Date of sample shipment (optional)
- Date of diagnosis of multiple myeloma
- Criteria for measurable disease
- Serum β2-microglobulin
- Serum albumin
- Eligibility criteria
Final analysis VRD randomization (Q2/Q3-2020)
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
