HOVON HO96 SCT
Main info
- Identifier:
- HOVON 96 GVHD SCT
- Sponsor:
- HOVON
- Working group party:
- SCT & Supportive care
- Age:
- 18-70
- Stage:
- 1st Line
- Included patients:
-
494(of 500)
- Active sites:
-
11(of 11)
- Title:
Prevention of severe GVHD after allogeneic hematopoietic stem cell transplantation, applied as consolidation immunotherapy in patients with hematological malignancies. A prospective randomized phase III trial.
Timeline
News
Inclusion of new patients closed per July 3th, 2018.
Flow
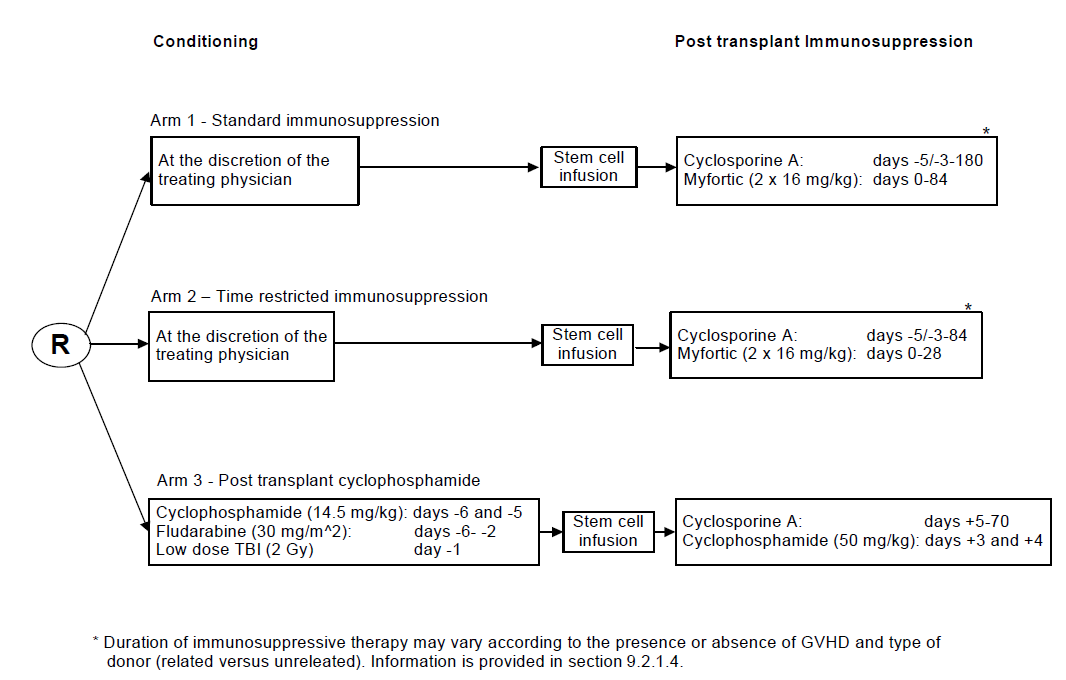
Details
- Phase:
- Prospective randomized Phase III study
- Monitoring Type:
- Not any more
- Objectives:
Objectives:
- to reduce the proportion of patients without GVHD within 180 days post-allo-SCT,
- to reduce the progression rate and
- to improve the progression free survival using a time restricted immunosuppressive regimen as compared to a prolonged, standard immunosuppressive regimen, or using a post-transplant regimen with high-dose cyclophosphamide.
- to asses the impact of allogeneic SCT on the quality of life in a prospectively treated cohortmof patients
Additional objective:
- to develop a predictive score, by means of clinical and laboratory parameters (using genomic and proteomic approaches) that allows for accurate identification of patients at high risk of severe GVHD as well as for identification of patients, who will not develop GVHD
Eligibility
- Inclusion Criteria:
- Age 18-70 inclusive
- AML, MDS, ALL, MM, CML, CLL, NHL, HL, or a myeloproliferative disease (MPD)
- Planned allogeneic stem cell transplantation
- Related or unrelated donor with a 8/8 HLA match (HLA A, B, C, DRB1)
- WHO performance status 0-2
- Written Informed Consent
- Negative pregnancy test (if applicable)
- Patients who are willing and capable to use adequate contraception during Myfortic treatment (all pre-menopausal women)
- Exclusion Criteria:
- Renal dysfunction (serum creatinine > 150 µmol/L or clearance < 50 ml/min)
- Patients with active, uncontrolled infection
- Cord Blood transplantation
- Patients receiving ATG pre-transplantation as part of the conditioning regimen
- Patients with progressive disease in case of MM, CLL, NHL, HL
- Patients with > 5% marrow blasts in case of AML, ALL, CML
- Patients with EMD in case of AML, ALL, CML
Registration Details
Eligible patients should be registered and randomized before start of treatment. Patients need to be registered at the HOVON Data Center of the Erasmus MC Rotterdam – Clinical Trial Center via the Internet or by phone call: +31.10.7041560 or fax +31.10.7041028 Monday through Friday, from 09:00 to 17:00 CET. A logon to TOP can be requested at the HOVON Data Center for participants.
The following information will be requested at registration:
- Protocol number
- Institution name
- Name of caller/responsible investigator
- Local patient code (optional)
- Sex
- Date of birth
- Date written informed consent
- Will the patient particpate in the QoL part of the trial (no longer applicable)
- Eligibility criteria (see 8.1)
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
