HOVON HO97 AML
Main info
- Identifier:
- HO97 AML/MDS
- Sponsor:
- HOVON
- Working group party:
- Leukemia
- Age:
- >= 60
- Stage:
- 1st Line
- Echelon:
- Level C-HIC
- Included patients:
-
118(of 126)
- Active sites:
-
26(of 19)
- Title:
Randomized maintenance therapy with Azacitidine (Vidaza) in older patients (>= 60 years of age) with acute myeloid leukemia (AML) and refractory anemia with excess of blasts (RAEB, RAEB-t)
Timeline
News
26OCT2016: 1 December 2016 the HOVON 97 study is going to be closed for the inclusion of new patients.
Please read the following letter form the PI, Prof. Dr. G.A. Huls:
Dear colleagues,
Thank you for your interest and support of the HOVON 97 study during the last years. Currently, 116 patients have been included. As discussed at the last HOVON leukemia group meeting, it has been decided to stop the study, mainly due to slow accrual and also because it has become clear that the number of events (especially after 1 year follow up) is low and therefore, the number of events as defined in the original statistical plan will not be reached (also not if more patients will be included).
In line with this we would like to announce that the HOVON 97 study will be closed for inclusion December 1st 2016. After additional follow up the study will be analyzed June/July 2017, to be able to submit an abstract to ASH 2017.
Best regards, Gerwin Huls
03JAN2012: The first amendment to the protocol has been approved by the central METC (UMCG). Please find the updated protocol below. Also the CRF instructions has been updated, for of the data management in favor of the interim analysis.
02DEC2011: An interim analyse has been planned for the first 40 included patients, in March 2013.
12APR2011: PLEASE FIND A NEW VERSION OF THE CRF and CRF instructions, SAE form and SAE instructions (see 6. Download documentation / forms)
23JUN2010: The HOVON 97 AML study has been approved for Belgium. Please find the documents for Belgium in the HO97 BELGIUM Portfolio (see Download documentation / forms - Belgium).
22JUN2010: A 'Verpleegkundig document' is available. This document is according to the UMCG directives. Please check this document before use.- 22JUN2010: A 'Verpleegkundig document' is available. This document is according to the UMCG directives. Please check this document before use.
18MAR2009: The HOVON 97 AML has been approved by the central METC (UMCG).
Flow
Details
- Phase:
- Prospective randomized Phase III study
- Monitoring Type:
- Not any more
- Objectives:
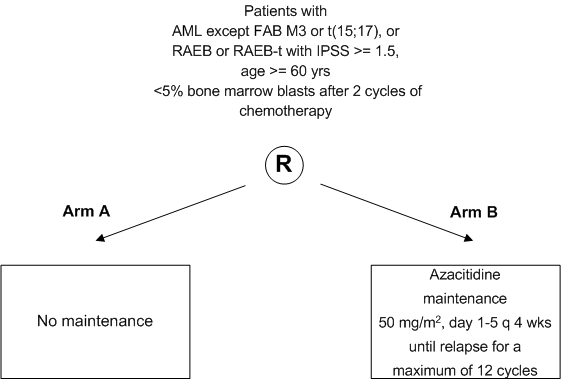
To assess, in a randomized study the value of Azacitidine as post remission therapy (in comparison to observation) in elderly patients with AML, RAEB or RAEB-t with respect to the disease free survival.
- In addition, post remission Azacitidine therapy will be evaluated with respect to toxicity, probability of relapse and probability of death in first CR and overall survival.
- To evaluate prognostic factors (e.g. phenotype, cytogenetics) in the context of post remission therapy with Azacitidine as regards to overall survival, and disease free survival.
Eligibility
- Inclusion Criteria:
- Age 60 years or more
- Subjects with a cytopathologically confirmed diagnosis of
- AML (M0-M2 and M4-M7, FAB classification, appendix A), or
- refractory anemia with excess of blasts (RAEB) or refractory anemia with excess ofblasts in transformation (RAEB-t) with an IPSS score of >1.5 (appendix C)
Note: Subjects with a secondary AML progressing from antecedent myelodysplasia and biphenotypic leukaemia are eligible.
- Less than 5% bone marrow blasts and absence of Auer rods after 2 cycles of induction therapy (this induction therapy can be according to HOVON81, HOVON92 or similar protocols. Of note: also patients who are treated according to one of these protocols but were not formally included in these studies are eligible for HOVON97)
- Hematological recovery, i.e. ANC ≥ 0.5 x 10^9/l and platelets ≥ 50 x 10^9/l
- WHO performance status ≤ 2 (see appendix F)
- Written informed consent
- Exclusion Criteria:
- Extramedullary disease
- Planned allogeneic hematopoietic cell transplantation
- Previous polycythaemia rubra vera
- Primary myelofibrosis
- Blast crisis of chronic myeloid leukemia
- AML-FAB type M3 or AML with cytogenetic abnormality t(15;17)
- Impaired hepatic or renal function as defined by:
- ALT and/or AST > 2.5 x normal value
- Bilirubin > 2 x normal value
- Serum creatinin > 2 x normal value (after adequate hydration)
- Concurrent severe and/or uncontrolled medical condition (e.g. uncontrolled diabetes, infection, hypertension, cancer, etc.)
- Cardiac dysfunction as defined by:
- Myocardial infarction within the last 6 months of study entry, or
- Reduced left ventricular function with an ejection fraction <50% as measured by MUGA scan or echocardiogram (another method for measuring cardiac function is acceptable)
- Unstable angina
- Unstable cardiac arrhythmias
Registration Details
Eligible patients should be randomized within 3 months after start of the second cycle of induction chemotherapy. Patients need to be registered at the HOVON Data Center of the Erasmus MC Rotterdam – location Daniel via the Internet via TOP (Trial Online Process; https://www.hdc.hovon.nl/top) or by phone call: +31.10.7041560 or fax +31.10.7041028 Monday through Friday, from 09:00 to 17:00 CET. A logon to TOP can be requested at the HOVON Data Center for participants.
The following information will be requested at registration:
- Protocol number
- Institution name
- Name of caller/responsible investigator
- Patient’s initials or code
- Sex
- Date of birth
- Date written informed consent
- Registered in other HOVON trial (HOVON 81, HOVON 92 or other)
- Stratification factors
- Specific items patient gives consent for (see ICF)
- Eligibility criteria
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
