HOVON HO101 CLL
Main info
- Identificatie:
- HO101 CLL
- Sponsor:
- HOVON
- Working group party:
- CLL
- Age:
- >= 18
- Stadium:
- 2de lijn
- Echelon:
- Level D
- Included patients:
-
0(of 532)
- Active sites:
-
19(of 24)
- Title:
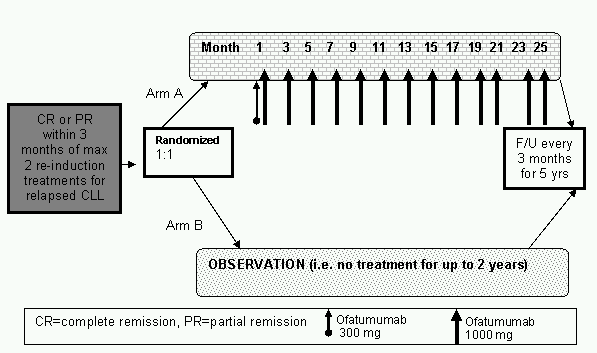
'Phase III Trial in Relapsed CLL Of a MonocLonal Antibody Ofatumumab
mainteNance therapy to delay proGression vs observation'
Timeline
News
The HOVON 101 CLL (PROLONG)/OMB112517 study has been closed for the inclusion of new patients.
Please note that this study applies to both 2nd and 3rd line treatment.
On the second item of the inclusion criteria, the confirmation by CT scan of the response is not necessary.
Flow
Details
- Phase:
- Prospective randomized Phase III study
- Monitoring Type:
- Objectives:
Primary objective:
- To evaluate PFS of subjects treated with ofatumumab maintenance treatment compared to no further treatment after remission induction in subjects with relapsed chronic CLL
Secondary objectives:
- To evaluate the improvement in response, improvement in response time to next CLL treatment and overall survival in subjects receiving ofatumumab maintenance compared to no further treatment
- To evaluate the PFS after next-line therapy and the time to progression after nextline therapy
- To evaluate the safety and tolerability in subjects with CLL receiving ofatumumab maintenance compared to no further treatment
- To evaluate the health-related quality of life in subjects with CLL receiving ofatumumab maintenance compared to no further treatment as assessed by changes in patient reported outcome (PRO) measures relative to baseline
- To evaluate prognostic marker correlation with clinical response in subjects with CLL receiving ofatumumab maintenance compared to no further treatment
- To evaluate ofatumumab pharmacokinetic parameters in subjects with CLL receiving maintenance ofatumumab every 2 months
Eligibility
- Inclusion Criteria:
- Adults with documented diagnosis of CLL based on the modified IWCLL updated NCI-WG guidelines [Hallek, 2008]
- At least PR according to the revised 2008 NCI-WG CLL criteria within 3 months of the response assessment after the last dose of 2nd/3rd line treatment
- The anti-leukemic treatment before study entry should have been for at least 3 months or 3 cycles
- ECOG Performance Status of 0-2
- Signed written informed consent prior to performing any study-specific procedures
- Exclusion Criteria:
- Known primary or secondary fludarabine-refractory subjects, defined as treatment failure (failure to achieve a CR or PR) or disease progression within 6 months [Hallek, 2008]
- Prior maintenance therapy
- Known transformation of CLL (e.g. Richter’s transformation), prolymphocytic leukemia (PLL), or CNS involvement of CLL
- Active Autoimmune Hemolytic Anemia (AIHA) requiring treatment except if in the opinion of the investigator it is thought not to affect the subject’s safety, the conduct of the study or the interpretation of the data
- Previous autologous or allogeneic stem cell transplantation
- Chronic or current active infectious disease requiring systemic antibiotics, antifungal, or antiviral treatment such as, but not limited to, chronic renal infection, chronic chest infection with bronchiectasis, tuberculosis and active Hepatitis B or C (Positive serology for Hepatitis B (HB) defined as a positive test for HBsAg. In addition, if negative for HBsAg but HBcAb positive (regardless of HBsAb status), a HBV DNA test will be performed and if positive the subject will be excluded.) If HBV DNA is negative, subject may be included but must undergo HBV DNA monitoring (see Section 6.3.4). Prophylactic antiviral therapy may be initiated at the
discretion of the investigator.
*Other past or current malignancy (with the exception of basal cell carcinoma of the skin or in situ carcinoma of the cervix or breast) unless the tumor was successfully treated with curative intent at least 2 years prior to trial entry except if in the opinionof the investigator it is thought not to affect the subject’s safety, the conduct of the study or the interpretation of the data
- Clinically significant cardiac disease including unstable angina, acute myocardial infarction within 6 months prior to screening, congestive heart failure, and arrhythmia requiring therapy, with the exception of extra systoles or minor conduction abnormalities except if in the opinion of the investigator it is thought not to affect the subject’s safety, the conduct of the study or the interpretation of the data
- History of significant cerebrovascular disease or event with symptoms or sequelae
- Significant concurrent, uncontrolled medical condition that in the opinion of the investigator contraindicates participation in this study
- Other anti-leukemic use of medications including glucocorticoids
- Known HIV positive
- Screening laboratory values:
- Platelets<50 x 10^9/L
- Neutrophils<1.0 x 10^9/L
- Creatinine > 1.5 times upper normal limit (unless normal creatinine clearance)
- Total bilirubin > 1.5 times upper normal limit (unless due to liver involvement of CLL or Gilbert’s syndrome)
- Alanine Aminotransferase (ALT) > 2.5 times upper normal limit (unless due to liver involvement of CLL)
- Alkaline phosphatase > 2.5 times upper normal limit
- Known or suspected hypersensitivity to ofatumumab that in the opinion of the investigator or medical monitor contraindicates study participation
- Subjects who have received treatment with any non-marketed drug substance or experimental therapy within 5-terminal half-lives or 4 weeks whichever is longer prior to first dose of study medication or currently participating in any other interventional clinical study
Note: Participation in any other interventional clinical study after disease progression during post PD follow-up is permitted
- Lactating women, women with a positive pregnancy test at Visit 1 or women (of childbearing potential) as well as men with partners of childbearing potential, who are not willing to use adequate contraception from study start through one year
following last ofatumumab dose. Adequate contraception is defined as abstinence, oral hormonal birth control, implants of levonorgestrel, estrogenic vaginal ring, percutaneous contraceptive patches, intrauterine device, and male partner sterilization if male partner is sole partner for that subject. For females in the USA, the use of a double barrier method is also considered adequate (condom or occlusive cap plus spermicidal agent).
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
