HOVON HO100 ALL
Main info
- Identificatie:
- HO100 ALL
- Sponsor:
- HOVON
- Working group party:
- Leukemia
- Age:
- 18-70
- Stadium:
- 2de lijn
- Echelon:
- Level C-HIC
- Included patients:
-
376(of 376)
- Active sites:
-
31(of 32)
- Title:
Clofarabine added to prephase and consolidation therapy in acute lymphoblastic leukemia in adults. A prospective randomized trial.
Timeline
News
Enter HO100 app: http://www.hovon100.nl/
20NOV2018:
Our faxserver (+31(0)107031201) at HOVON Data Center (HDC) will no longer be used.
This means the HDC cannot receive any forms e.q. SAE forms/pregnancy forms via this faxserver anymore.
From now on these forms can be send to the e-mail address: saereport@erasmusmc.nl. See also adjusted SAE form /pregnancy form.
24JAN2017:
The reporting of Safety forms in the HO100 study has changed. From now one you must send to new faxnumber +31(0)10 7031201. See also adjusted SAE form /pregnancy form.
07NOV2016: HOVON 100 study closed for further patient inclusion
The HOVON 100 ALL/EORTC 06083 trial is closed as of 07-NOV-2016 for the inclusion of new patients. The trial is closed because it has reached its target of 376 patients. The patients that were included should be treated and followed according to protocol. We kindly ask you to send in send in the treatment data and Follow Up data per CRF to the HOVON Data Center. Also remember to send in the data/material for the side studies and central reviewers. We would like to thank you for your enthusiasm. Only with your participation were we able to reach this milestone.
07SEPT2016: HOVON100 patient screening list” for the last HO100 patients
Important: “HOVON100 patient screening list” for the last HO100 patients
If you have a patient in screening for the HO100 study, please inform HDC. Your patient will be added to the “HOVON100 patient screening list” and we will inform you about how many patients at that moment are in screening.
Please provide in this email:
- the date of birth
- the planned date when expected to register the patient in the HO100
- the planned start date for the first HO100 cycle.
Only patients listed on the “HO100 patient screening list” who are eligible will actually be registered by us.
Because there are 30 sites open for inclusion, no registration places can be reserved. If the list of patients in screening becomes longer than patients that can still be registered no more patients will be listed on the screening list.
Important: Please inform us as soon as possible if the (your) patient listed on the screening list turns out not to be eligible during screening for the HO100, so we can delete the listed patient from the “HOVON100 patient screening list”.
Important: Registration the last HO10O patients via TOP
Patient registration in TOP will be exclusively done via the HOVON Data Center (HDC). Participating sites can no longer register a patient in TOP. For registering a patient you must complete the HOVON100 MM registration form and fax it to the HOVON Data Center.
Note: Only patients listed in “HOVON100 patient screening list” who are eligible will actually be registered by us.
08AUG2016: Gewijzigde medicatie bestelformulieren en Pharmacy Info letter
Er zijn vanaf heden 8 AUGUSTUS enkele wijzigingen in de bestel procedures van de HO100 medicatie; Clofarabine en PEG-Asparaginase (Oncaspar)
HO1O0 Clofarabine medicatie:Het e-mail adres is gewijzigd voor het bestellen van clofarabine ; de bestelformulieren moeten gemailed worden naar CTSRequests-TGR@pciservices.com
HO100 PEG-Asparaginase (Oncaspar): De bestelformulieren van PEG-Asparaginase (Oncaspar) zijn gewijzigd (Komtur Pharmaceuticals Berlin)
Het e-mail adres is gewijzigd voor het bestellen van medicatie; de PEG-Asparaginase bestelformulieren moeten gemailed worden naar oncaspar@komtur.com
De artsenverklaring moet nog bij elke bestelling meegestuurd worden.
07APR2015: SKION Netherlands new address: Zinkwerf 5-7, 2544 EC THE HAGUE. From 15 April 2015, send MRD samples to the new address.
07APR2015: lab Investigations NL and MRD logistics NL has been updated
28OCT2014: Info Pharmacy letter and order form PEG-L-asparaginase has been updated
10JUN2014: Amendment 13 (for Dutch and Belgian sites only); protocol version 27-MAR-2014 valid
05JUL2012: Current phase: III; Current dose level clofarabine: 30 mg/m2
Flow
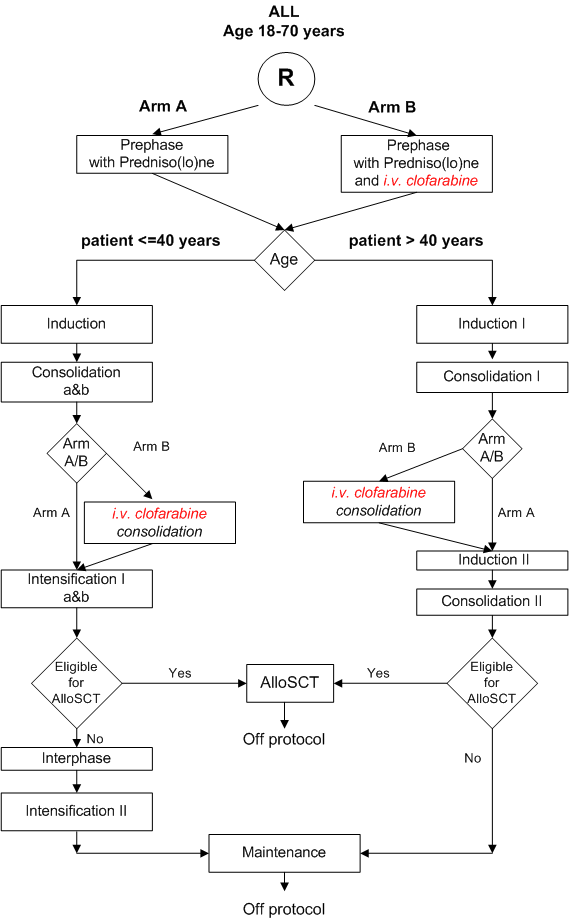
Details
- Phase:
- Prospective Phase II/III study
- Monitoring Type:
- Not any more
- Objectives:
Phase II part
- To determine the feasibility of adding i.v. clofarabine to standard prephase therapy (followed by induction chemotherapy)
Phase III part
Primary objective:- To improve EFS in adult ALL patients by the addition of i.v. clofarabine to prephase and consolidation therapy
Secondary objectives:
- To improve the molecular response rate of adult ALL following RI by the addition of i.v. clofarabine to standard prephase and consolidation therapy
- To improve DFS, and OS in adult ALL patients by the addition of i.v. clofarabine to the standard prephase and consolidation therapy
- To document safety and toxicity of adding clofarabine to standard prephase and consolidation therapy in adult ALL
- To assess and compare clinical outcome of patients with and without an HLA-identical sibling in a donor vs no-donor analysis
- To determine the incidence and clinical relevance of thromboembolic (TE) complications during adult ALL treatment with LMWH as antithrombotic prophylaxis
- To determine the influence of ALL (treatment) on different coagulation markers
Eligibility
- Inclusion Criteria:
- Patients aged 18 to 70 years inclusive
- Primary previously untreated B or T-lineage ALL (excluding ALL with mature B-cell phenotype, but including Philadelphia positive or BCR-ABL positive ALL) or previously untreated T-LBL (pretreatment with prednisolone for 7 days is allowed)
- WHO performance status 0 – 2
- Adequate renal and hepatic function tests as indicated by the following laboratory values:
- Serum creatinine ≤1.0 mg/dl (≤ 88.7 micromol/L); if serum creatinine >1.0 mg/dl (>88.7 micromol/L), then the glomerular filtration rate (GFR) must be >60 ml/min/1.73 m2 as calculated by the Modification of Diet in Renal Disease equation where the predicted GFR
(ml/min/1.73 m2) = 186 x (Serum Creatinine in mg/dl)-1.154 x (age in years)-0.023 x (0.742 if patient is female) x (1.212 if patient is black)
NOTE: if serum creatinine is measured in micromol/L, recalculate it in mg/dl according tobthe equation: 1 mg/dl = 88.7 micromol/L) and used above mentioned formula.- Serum bilirubin ≤ 1.5 × upper limit of normal (ULN)
- Aspartate transaminase (AST)/alanine transaminase (ALT) ≤ 2.5 × ULN
- Alkaline phosphatase ≤ 2.5 × ULN
- Negative pregnancy test at inclusion, if applicable
- Written informed consent
- Exclusion Criteria:
- Mature surface Ig positive B-cell leukemia/lymphoma
- Acute undifferentiated leukemia
- Severe cardiovascular disease (arrhythmias requiring chronic treatment, congestive heart failure or symptomatic ischemic heart disease)
- Severe pulmonary dysfunction (CTCAE grade III-IV, see appendix D)
- Severe neurological or psychiatric disease
- History of active malignancy during the past 5 years with the exception of basal carcinoma of the skin or stage 0 cervical carcinoma
- Active, uncontrolled infection
- Patient known to be HIV-positive
- Patient is a lactating woman
- Any psychological, familial, sociological and geographical condition potentially hampering compliance with the study protocol and follow-up schedule
- Unwilling or not capable to use effective means of birth control (all men, all premenopausal women under the age of 50 need contraception for two years after the last period, and women older than 50 yrs for at least one year)
Registration Details
Patients need to be registered at the HOVON Data Center, Erasmus MC Cancer Institute, Clinical Trial Center via the Internet via TOP
(Trial Online Process; https://www.hdc.hovon.nl/top) or by phone call: +31.10.7041560 or fax +31.10.7041028 Monday through Friday, from 09:00 to 17:00 CET. A logon to TOP can be requested at the HOVON Data Center for participants.
The following information will be requested at registration:
- Protocol number
- Institution name
- Name of caller/responsible investigator
- Patient’s initials or code
- Sex
- Date of birth
- Date written informed consent
- Eligibility criteria
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
