HOVON HO104 MM
Main info
- Identificatie:
- HO104 AL Amyloidosis
- Sponsor:
- HOVON
- Working group party:
- Myeloma
- Age:
- 18-70
- Stadium:
- 1st lijn
- Echelon:
- Limited Site Selection
- Included patients:
-
57(of 114)
- Active sites:
-
20(of 22)
- Title:
A multicenter, prospective study of bortezomib and dexamethasone as induction
treatment followed by high dose melphalan (HDM) and autologous stem cell
transplantation (SCT) in patients with de novo amyloid light chain (AL) amyloidosis.
Timeline
News
29JAN2021: Last patient's last visit. Close-outs and closure of study are in progress.
01APR2016: The study is closed for inclusion of new patients because the target number has been reached. Included patients will be followed until 5 years after registration.
21JUL2015: Please note that the target of study patients is 57 (50 patients registered for Bortezemib, 7 'arm A' patients of the primary phase III study). Sites will be notified by email once the study is almost closed.
2012: Please be aware that there is a restriction on the number of sites that can participate in this study, due to study-specific monitoring. At the moment, no extra sites can be added. If you have any questions, please contact the HOVON Data Center.
New documents:
22MAR2017: new version CRFs (v7)
15JUL2015: All mentioned documents are updated in relation to amendment 05
- Protocol (clean + summary of changes)
- ICF (clean + summary of changes)
- Pre-study ICF
- Echo review information
- Belgium specific portfolio
Flow
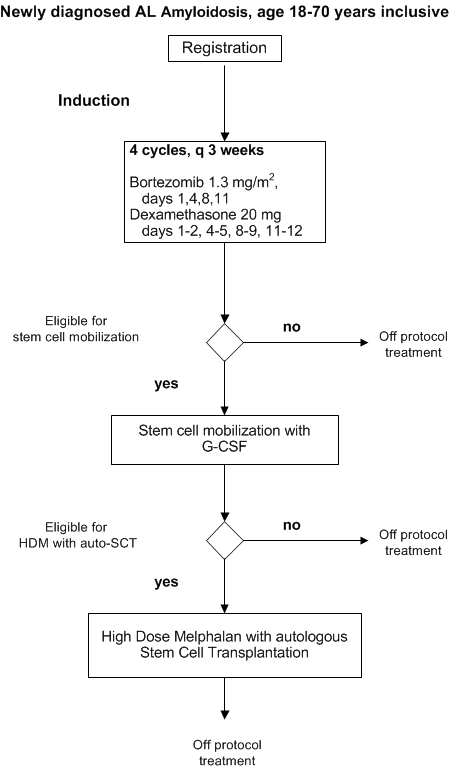
Details
- Phase:
- Prospective Phase III study
- Monitoring Type:
- Study Specific
- Objectives:
- To determine the efficacy of bortezomib plus dexamethasone induction therapy followed by HDM and auto-SCT in patients with newly diagnosed AL amyloidosis who are 18-70 years inclusive.
- To asses the safety of bortezomib plus dexamethasone as induction treatment followed by HDM and auto-SCT in patients with newly diagnosed AL amyloidosis who are 18-70 years inclusive.
Eligibility
- Inclusion Criteria:
- Biopsy proven, systemic, untreated AL amyloidosis requiring systemic chemotherapy,
- Age 18 -70 years inclusive at the time of signing the informed consent form,
- Measurable plasma cell dyscrasia, defined as a detectable M-protein with serum electrophoresis and/or level of involved FLC > 50 mg/L,
- Life expectancy > 3 months,
- WHO performance status 0-2,
- NYHA stage 1-2,
- Negative pregnancy test at inclusion for women of childbearing potential,
- Written informed consent.
Inclusion criteria for stem cell mobilization and HDM with auto-SCT
Inclusion criteria- Life expectancy > 3 months,
- WHO performance status 0-2,
- NYHA stage 1-2,
- Cardiac ejection fraction > 45% (only before HDM),
- Negative pregnancy test in female patients of childbearing potential.
- Exclusion Criteria:
- Multiple Myeloma stage II and III (Durie and Salmon, see appendix K),
- Any serious medical condition, laboratory abnormality, or psychiatric illness that would prevent the subject from signing the informed consent form,
- Any psychological, familial, sociological and geographical condition potentially hampering compliance with the study protocol and follow-up schedule,
- Previous treatment for plasma cell dyscrasia,
- Pregnant or breast feeding females,
- Presence of other active malignancy or a history of active malignancy during the past 5 years, with the exception of nonmelanoma skin cancer, stage 0 cervical carcinoma, or treated early-stage prostate cancer provided that prostate-specific antigen is within normal limits,
- Hypersensitivity to boron or mannitol,
- Uncontrolled infection,
- Symptomatic orthostatic hypotension defined as a decrease in systolic blood pressure on standing of >20 mmHg combined with symptoms like dizziness, cerebral and/or cardial ischemia,
- Symptomatic effusions, defined as pleural effusion or ascites needing drainage therapy,
- NT pro BNP level > 5000 pg/ml and Troponin T> 0.06 microgram/l (not high senstitivity assay) or NT proBNP level > 5000 pg/ml and Troponin I > 2 times ULN
- Positive for HIV or infectious hepatitis, B or C (screening obligatory),
- Bilirubin > 2x upper limit of normal,
- Creatinine clearance < 30 ml/min (after rehydration),
- Absolute neutrophil count < 1.0 × 10^9/L,
- NCI CTCAE grade peripheral sensory neuropathy > grade 2,
- NCI CTCAE grade peripheral sensory neuropathy > grade 1 in the presence of neuropathic pain,
- NCI CTCAE grade peripheral motor neuropathy > grade 2
- Concurrent diagnosis of B-cell NHL or B-CLL,
- Previous organ transplantation,
- Unwilling or unable to use adequate contraception
Exclusion criteria for stem cell mobilization and HDM with auto-SCT:
- Symptomatic effusions, defined as pleural effusion or ascites needing drainage therapy,
- Uncontrolled infection,
- Symptomatic orthostatic hypotension defined as a decrease in systolic blood pressure on standing of >20 mm Hg combined with symptoms like dizziness, cerebral and/or cardial ischemia. Anti-hypotensive medication is allowed,
- Absolute neutrophil count < 1.0 × 10^9/L,
- Bilirubin > 2x upper limit of normal.
- Stem cell mobilization started > 12 weeks after the start of the last course of bortezomib and dexamethasone
Registration Details
Eligible patients should be registered before start of treatment. Patients need to be registered at the HOVON Data Center by one of the following options:
- Trial Online Process (TOP, https://www.hdc.hovon.nl/top). A logon to TOP can be requested at the HOVON Data Center for participants.
- By faxing the completed registration/randomization CRF +31.10.7041028 Monday through Friday, from 09:00 to 17:00 CET
- By phone +31.10.7041560 Monday through Friday, from 09:00 to 17:00 CET
The following information will be requested at registration:
- Protocol number
- Institution name
- Name of caller/responsible investigator
- Local patient code (optional)
- Sex
- Date of birth and “Age in years”
- Date written informed consent
- Specific items patient gives consent for (see ICF)
- Eligibility criteria
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
