HOVON HO106 SCT
Main info
- Identificatie:
- HO106 SCT
- Sponsor:
- HOVON
- Working group party:
- SCT & Supportive care
- Age:
- 18-65
- Stadium:
- 2de lijn
- Echelon:
- Level A
- Included patients:
-
60(of 61)
- Active sites:
-
6(of 5)
- Title:
'A phase II study to assess engraftment and engraftment kinetics after double cord
blood transplantation with a reduced-intensity conditioning regimen in patients
eligible for allogeneic stem cell transplantation lacking a matched unrelated donor.'
Timeline
News
This study has been closed for further inclusion on 20 januari 2012
Flow
Details
- Phase:
- Prospective Phase II study
- Monitoring Type:
- Not any more
- Objectives:
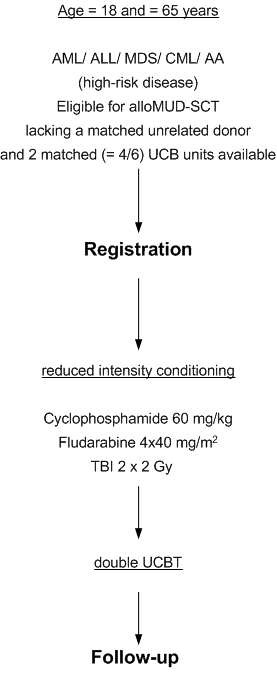
- To assess engraftment and engraftment kinetics after double cord blood transplantation preceded by a reduced-intensity conditioning regimen in patients eligible for allogeneic stem cell transplantation lacking a matched unrelated donor.
- To evaluate immune reconstitution, acute and chronic GVHD, chimerism, toxicity, time to treatment failure, progression-free survival and overall survival after double unit UCBT.
- To study patient-versus-graft, graft-versus-patient and graft-versus-graft interactions
Eligibility
- Inclusion Criteria:
Age 18-65 years inclusive
- Meeting the criteria for a MUD allo SCT and high risk disease (see below)
- Lacking a sufficiently matched volunteer unrelated donor or lacking such a donor within the required time period of ≤ 2 months in case of urgently needed alloSCT
- Availability of 2 sufficiently matched UCB grafts with a total nuclear cell count > 4 x 10^7/kg
- WHO performance status ≤ 2
- Written informed consent
High risk disease as defined by:
- AML with -5, -7, EVI-1-expression or complex karyotype in first CR
- Relapsed AML/ MDS in second or subsequent CR
- ALL with t(9;22), t(4;11), t(1;19) or with high WBC at diagnosis (B-ALL > 30x10^9/l, T-ALL > 100x10^9/l) in first CR, or no CR after first induction but in CR after rescue chemotherapy
- Relapsed ALL in second or subsequent CR
- CML in second chronic phase after treatment for CML blast crisis
- VSAA or SAA relapsing after or failing immunosuppressive therapy
Patients with the following diseases may be included if considered high risk disease:
- Relapse AML with t(8;21) or inv16 in second or subsequent CR, with poor risk according to Breems prognostic score (appendix A)
- AML/MDS in patients 61-65 years inclusive, in first CR
- CML in second chronic phase after treatment for acceleration phase
- Lymphocytoplasmacytoid lymphoma, responsive disease after at least third line chemotherapy
- Folliculair NHL, responsive disease after at least third line chemotherapy
- CLL, responsive disease after at least third line chemotherapy
- Exclusion Criteria:
- Relapse APL
- Primary myelofibrosis
- Bilirubin and/or transaminases > 2.5 x normal value
- Creatinine clearance < 40 ml/min
- Cardiac dysfunction as defined by:
- Reduced left ventricular function with an ejection fraction < 45% as measured by MUGA scan or echocardiogram (another method for measuring cardiac function is acceptable)
- Unstable angina
- Unstable cardiac arrhythmias
- Pulmonary function test with VC, FEV1 and/ or DCO < 50%
- Active, uncontrolled infection
- History of high dose total body irradiation
- HIV positivity
Registration Details
Eligible patients should be registered before start of treatment. Patients need to be registered at the HOVON Data Center by one of the following options:
- Trial Online Process (TOP, https://www.hdc.hovon.nl/top). A logon to TOP can be requested at the HOVON Data Center for participants.
- By faxing the completed registration/randomization CRF +31.10.7041028 Monday through Friday, from 09:00 to 17:00 CET
- By phone +31.10.7041560 Monday through Friday, from 09:00 to 17:00 CET
The following information will be requested at registration:
- Protocol number
- Institution name
- Name of caller/responsible investigator
- Patient’s name code
- Sex
- Date of birth
- Date written informed consent
- Specific items patient gives consent for (see ICF)
- Eligibility criteria
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
