HOVON HO109 CLL
Main info
- Identificatie:
- HO109 CLL
- Sponsor:
- HOVON
- Working group party:
- CLL
- Age:
- >= 18
- Stadium:
- 1st lijn
- Echelon:
- Level D
- Included patients:
-
63(of 62)
- Active sites:
-
35(of 37)
- Title:
Efficacy and safety of first-line therapy with chlorambucil, rituximab and lenalidomide (Revlimid®) (CR2) in elderly patients and young frail patients with advanced Chronic Lymphocytic Leukemia (CLL): a phase I/II trial.
Timeline
News
New version of lab manual including time point progressive disease: Version 6, 14 February 2017, including time point progressive disease, is now in place.
18AUG2015: Study is closed for inclusion of new patients
17AUG2015: Reminder: Please call HDC in case of a possible patient for this study
Last patient will probably be entered this week. (for further information see news of 27-7-2015 below).
27JUN2015: Registration of last three patients via HOVON Data Center
This is to prevent the situation where a patient has been informed and invited to participate but cannot be registered anymore because of closure of the study in the time needed for the informed consent, the following change in informing patients and registration will be applied.
Patient diary for Lenalidomide available
11MAR2015: Amended documents available:
New version of protocol, ICF and CRFs available for implementation.
New version of lab manual : Version 5, 7 August 2014 is now in place.
Stop collection of samples for coagulation side study
April 2014: the collection of samples for the coagulation side study will stop, because it has proven to be not feasible for many participating hospitals. A updated labmanual is available from this website.
Start of fase II: Since 7NOV2013 fase II is open for inclusion of new patients.
Dose level: Vanaf 1 maart 2013 worden nieuwe patiënten geincludeerd in dose level 2.
Gewijzigde lenalidomide bestelling en levering: Vanaf 1 januari 2013 wordt overgegaan op lenalidomide levering in blisterverpakking via pooled supplies. De levertijd is 2 weken.
Het bestelformulier en de informatiebrief voor de apothekers is aangepast.
With the current inclusion rate we expect that the study will be closed for entry of new patients in September/October 2015. From now on patients for the HOVON 109 study will be entered via the HOVON Data Center instead of directly via TOP. Please inform HOVON Data Center (HDC) if you have a patient that will be informed about the HOVON 109 CLL study. These patients will be put on a list. When, after screening, the patient is eligible, HOVON Data Center is to be called to see whether it is still possible to register the patient in study.
Flow
Details
- Phase:
- Prospective Phase I/II study
- Monitoring Type:
- Not any more
- Objectives:
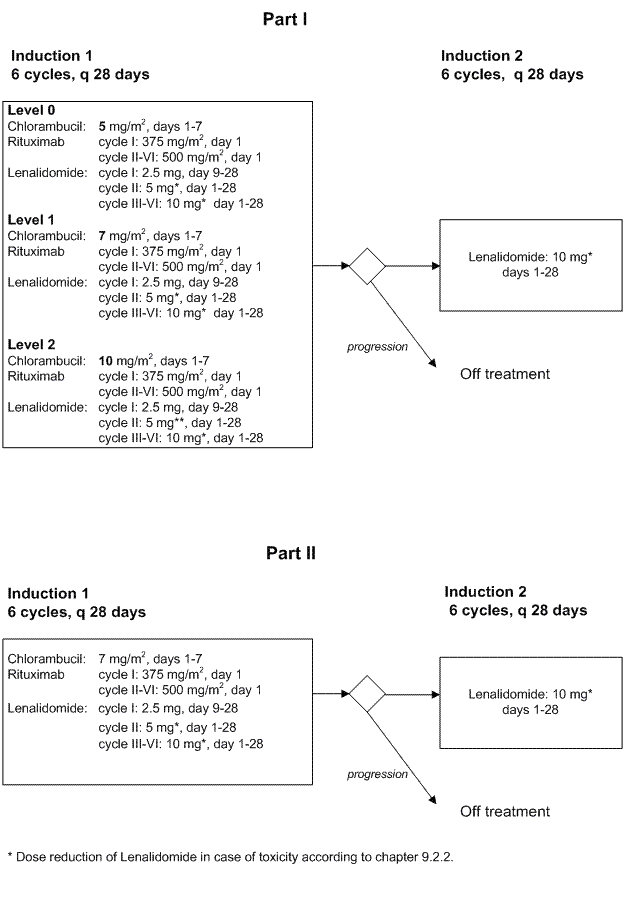
Part I
Primary objective:- To determine the maximum tolerated dose (MTD) and recommended part II dose level (RDL) of Chlorambucil when combined with Rituximab and Lenalidomide in a 28-days schedule. See paragraph 13 for definitions of MTD and RDL
Secondary objectives:
- To evaluate toxicity, especially tumor lysis syndrome (TLS), tumor flare reaction (TFR) and clinically relevant hematologic toxicityPart II
Primary objective:- To investigate the efficacy of a maximum of 6 cycles of Chlorambucil with Rituximab plus Lenalidomide at the RDL, as determined by the CR+PR rate
Secondary objectives:
- To evaluate the efficacy of Lenalidomide monotherapy in patients without progressive disease after 6 cycles of CR2
- To evaluate toxicity, especially tumor lysis syndrome (TLS), tumor flare reaction (TFR) and clinically relevant hematologic toxicity
- To evaluate progression free survival
- To evaluate event-free survival
- To evaluate overall survival
Eligibility
- Inclusion Criteria:
Diagnosis of CLL without prior treatment;
- Patients with symptomatic (according to IWCLL guidelines56 ) Binet stage A / Rai stage 0 or Binet stage B or C / Rai I, II, III or IV (appendix A);
- Age 65 - 80 years, inclusive, at the time of signing the informed consent form, or age 18 – 64, inclusive, and CIRS ≥ 7 (appendix E);
- Able to adhere to the study visit schedule and other protocol requirements;
- WHO performance status of ≤ 2;
- Laboratory test results within these ranges: absolute neutrophil count ≥ 1.0 x 10^9/l, platelet count ≥ 30 x 10^9/l, creatinine clearance ≥ 60 ml/min, total bilirubin ≤ 25 μmol/L, AST & ALT ≤ 2 x ULN; in case the estimated creatinine clearance is too low (≥40, <60 ml/min) one may determine the Glomular Filtration Ratio (GFR) from creatinine by 24 hours urine collection. This should be ≥ 60 ml/min ,
- Females of childbearing potential must have a negative serum or urine pregnancy test within 10 - 14 days prior to and again within 24 hours of starting lenalidomide;
- Patients who are willing and capable to use adequate contraception during the therapy (all men, all women of childbearing potential). Patients must be able to adhere to the requirements of the Lenalidomide Pregnancy Prevention Risk Management Plan;
- Written informed consent.
- Exclusion Criteria:
- Patients that are unable or unwilling to adhere to the requirements of the Lenalidomide Pregnancy Prevention Risk Management Plan;
- Intolerance of exogenous protein administration;
- Hepatitis B Ag positive, Hepatitis C positive and/or HIV positive patients;
- Patients with uncontrolled Autoimmune Hemolytic Anemia (AIHA) or autoimmune thrombocytopenia (ITP);
- Active fungal, bacterial, and/or viral infection;
- Pregnant or breast-feeding females (lactating females must agree not to breast feed while taking lenalidomide);
- Use of any other experimental drug or therapy within 28 days of baseline;
- Known hypersensitivity and/or serious adverse reactions to lenalidomide or similar drugs;
- Any prior use of lenalidomide;
- Concurrent use of other anti-cancer agents or treatments;
- Uncontrolled hyperthyroidism or hypothyroidism;
- Patients with history of idiopathic deep venous thrombus and/or pulmonary embolism within last three years;
- Neuropathy ≥ grade 2;
- History of active malignancy during the past 5 years with the exception of basal carcinoma of the skin; squamous cell carcinoma of the skin, carcinoma in situ of the cervix, carcinoma in situ of the breast, prostate cancer (TNM stage of T1a or T1b)
- Current inclusion in other clinical trials;
- Any psychological, familial, sociological and geographical condition potentially hampering compliance with the study protocol and follow-up schedule.
Registration Details
Eligible patients should be registered before start of treatment. Patients need to be registered at the HOVON Data Center by one of the following options:
- Trial Online Process (TOP, https://www.hdc.hovon.nl/top). A logon to TOP can be requested at the HOVON Data Center for participants.
- By faxing the completed registration CRF +31.10.7041028 Monday through Friday, from 09:00 to 17:00 CET
- By phone +31.10.7041560 Monday through Friday, from 09:00 to 17:00 CET
Patients for part I of the trial can only be registered by phone of by fax.
The following information will be requested at registration:
- Protocol number
- Institution name
- Name of caller/responsible investigator
- Local patient code (optional)
- Sex
- Date of birth
- Date written informed consent
- Eligibility criteria
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
