HOVON HO120 NHL
Main info
- Identificatie:
- HOVON 120 NHL (ZEST)
- Sponsor:
- HOVON
- Included patients:
-
474(of 474)
- Active sites:
-
18(of 1)
- Title:
A Phase 3, open-label, multicenter, randomized study of sequential Zevalin (ibritumomab tiuxetan) versus observation in patients at least 60 years of age with newly diagnosed diffuse large B-cell lymphoma in PET-negative complete remission after R-CHOP or R-CHOP-like therapy.
Timeline
News
For the Netherlands, HOVON is the co-sponsor of the HOVON 120 NHL /ZEST study.
Conduct of the study (site initiation, monitoring etc) will be performed by EMAS Pharma and will be the primary contact.
The HOVON Data Center can be contacted in case of questions about agreements and compensations.
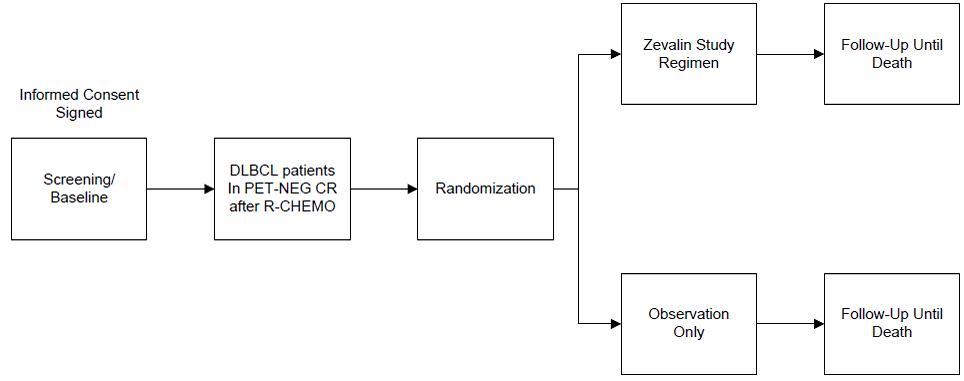
Flow
Details
- Phase:
- Prospective randomized Phase III study
- Monitoring Type:
- Objectives:
To evaluate the efficacy and safety of Zevalin compared with observation alone in patients
who are in PET-negative complete remission (CR) after first-line R-CHOP or R-CHOP like therapy.
Eligibility
- Inclusion Criteria:
- Patient is 60-years of age or older at time of randomization
- Histologically confirmed Ann Arbor stage II, III, or IV DLBCL; or FL Grade 3B according to the WHO classification 2008 (from initial diagnosis made prior to starting R-chemotherapy).
- An H&E stained slide and unstained slides must be available for confirmatory pathology review, as per the separate Pathology Guidance document. Patients may be randomized based on the local diagnosis.
- Presence of at least one IPI risk factor. The aaIPI is defined by one point for each factor:
- a. LDH > upper limit of normal (ULN);
- b. Stage III or IV; and
- c. WHO/ECOG performance status >1.
- First-line treatment must have been 6 cycles of standard R-CHOP or R-CHOP-like chemotherapy (e.g. R-CHOP21, R-CHOP14, or DA-EPOCH-R). Patients who received pre-phase therapy for the purpose of improving performance status prior to initiating RCHOP are eligible. Standard dose reductions for toxicity are allowed.
- Complete remission (CR) according to the Revised Response Criteria for Malignant Lymphoma (Appendix 2) after first-line treatment.
- a. Diagnostic CT scans with contrast of chest, abdomen, and pelvis must have been performed within 8 weeks after the first dose of the last cycle of R-chemotherapy. PET-CTs obtained elsewhere before and after R-CHOP are acceptable for evaluating response to R-CHOP, but a diagnostic CT prerandomization is requested as reference for post randomization interval change. A neck CT will be applicable if the patient had involvement of the neck region by palpation / physical examination at initial diagnosis. The CT portion of an FDG PET that includes the neck will be acceptable if the neck had involvement.
- b. A negative FDG-PET scan performed within 8 weeks after the first dose of the last cycle of R-chemotherapy and confirming CR, with negative defined as a score of 1-3 on the Deauville 5-point scale (Appendix 3) used to quantify radionucleotide density in PET scans as determined locally [23]. PET positive/indeterminate lesions which are confirmed on biopsy to harbor no active lymphoma will be considered negative for determination of CR status.
- c. If positive bone marrow involvement at initial diagnosis the patient must have a negative bone marrow biopsy following R-chemotherapy to confirm the CR.
- WHO/ECOG performance status of 0, 1 or 2.
- Adequate hematopoietic functions unsupported by transfusion within the last 2 weeks:
- Absolute neutrophil count (ANC) ≥ 1.5 x 10^9/L,
- Hemoglobin (Hgb) ≥ 9 g/dL
- Platelets ≥ 100 x 10^9/L.
- Patients with blood counts close to recovery towards these values after RCHOP should be discussed with the study Medical Monitor prior to randomization, but blood counts must have met these thresholds prior to treatment with Y-90 Zevalin.
- In patients who had a post R-chemotherapy bone marrow biopsy performed, the marrow must show cellularity > 15%. For patients without a post R-chemotherapy bone marrow biopsy (i.e. those patients with negative marrow at diagnosis), a repeat biopsy to assess bone marrow cellularity of > 15% will be required only for patients randomized to the Zevalin Regimen.
- Life expectancy of 6 months or longer.
- Written informed consent obtained according to local guidelines.
- Exclusion Criteria:
- Presence of any other malignancy or history of prior malignancy within 5 years of study entry. Within 5 years, patients treated with curative intent for Stage I or II cancers are eligible provided they have a life expectancy of > 5 years. The 5-year exclusion rule does not apply to-non melanoma skin tumors and in situ cervical cancer.
- Prior radioimmunotherapy, including radiation therapy for NHL, or any other NHL therapy.
- Presence of central nervous system (CNS) involvement, or testicular lymphoma at first diagnosis.
- DLBCLas histological transformation of previously diagnosed indolent B-cell lymphoma. Patients with De Novo Transformed DLBCL, defined as DLBCL on lymph node biopsy and a “discordant marrow” with small cells at initial diagnosis, are eligible.
- Known seropositivity for hepatitis C virus (HCV) or hepatitis B surface antigen (HbsAg). Patients who are positive for HbsAg but without active disease (Hep B PCR below the limits of detection) and who receive adequate prophylaxis may be enrolled, but should continue prophylaxis for at least 6 months after the last dose of rituximab or Zevalin.
- Known history of HIV infection.
- Abnormal liver function: total bilirubin > 2 × ULN unless secondary to Gilbert disease.
- Abnormal renal function: serum creatinine > 2.0 × ULN.
- Ongoing toxic effects of chemotherapy > grade 2 and expected to interfere with Zevalin treatment.
- Known hypersensitivity to murine or chimeric antibodies or proteins.
- Colony stimulating factor therapy administered more than 8 weeks after last dose of Rchemotherapy or within 4 weeks prior to planned administration of Zevalin.
- Concurrent severe and/or medically uncontrolled disease (e.g. uncontrolled diabetes, congestive heart failure, myocardial infarction within 6 months of study, unstable and uncontrolled hypertension, chronic renal disease, or active uncontrolled infection) which could compromise participation in the study.
- Treatment with investigational drugs less than 4 weeks prior to randomization.
- Major surgery less than 4 weeks prior to randomization.
- Concurrent systemic corticosteroid use for any reason except as premedication in case of known or suspected allergies to contrast media or as premedication for potential side effects of rituximab treatment. Patients on a chronic dose of prednisone for a medical condition (e.g. Asthma or autoimmune disease) less than or equal to 20mg daily, stable
for 4 weeks, are permissible.
- Unwillingness or inability to comply with the protocol.
- Pregnant women or women who are breastfeeding
Registration Details
Randomization via a web-based Inventory Management and Randomization System (IMRS).
