HOVON HO123 MM
Main info
- Identificatie:
- HO123 MM
- Sponsor:
- HOVON
- Working group party:
- Myeloma
- Age:
- >= 75
- Stadium:
- 1st lijn
- Echelon:
- Level D
- Included patients:
-
240(of 240)
- Active sites:
-
67(of 64)
- Title:
Feasibility and Efficacy of dose adjusted Melphalan – Prednisone – Bortezomib (MPV) in elderly patients ≥ 75 years of age with newly diagnosed Multiple Myeloma; a non-randomised phase II study
Timeline
News
25 August 2017: Protocol version 7 (9 august 2017) is active
2018: New link to eCRF: https://aleaclinical.com/hovon/dm/
Updated documents:
28-11-2017: New version of SAE report form and pregnancy form (notifications by mail instead of fax)
29-03-2016: New version of the flowsheet is available
15-jan-2016: ICF v6.1 and new insurance certificate are available
28-oct-2015: new 'Statement of Expenses' form is available
14-jul-2015: Protocol version 4 (22jan2015); Patient Information version 5 (18may2015) and Pre-Study Patient Information (15jun2015) are available
12-oct-2015: newsletter 8 is available
23-jan-2015: HOVON 123 MM eCRF and instructions are available
17-jun-2014: new TNT shipment instructions for bone marrow and blood
10-apr-2014: FAQ document is available
18-mar-2014: newsletter 4 & CT-scan shipment information form
20-feb-2014: QoL questionnaires (paper version) are available
11-dec-2013: Statement of Expenses form is available
11-dec-2013: Contactform & ITF labels are available
20-nov-2013: Updated version WMO certificate available
Flow
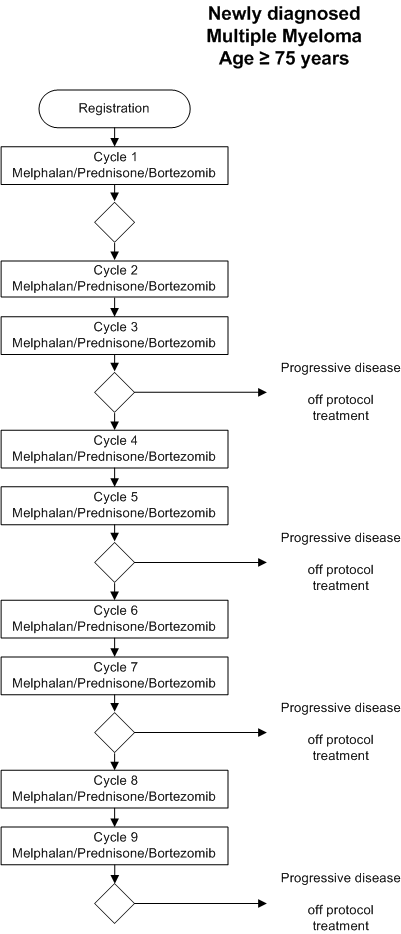
Details
- Phase:
- Prospective Phase II study
- Monitoring Type:
- Not any more
- Objectives:
Primary objective
- To assess the feasibility, defined as the discontinuation rate, of a dose-adapted MPV scheme in MM patients ≥ 75 years of age
Secondary objectives
- To assess toxicity
- To assess efficacy, defined as response rate, PFS and OS
- To assess time to response
- To assess the relative dose intensity of MPV
- To assess the cumulative dose intensity of MPV
- To assess the predictive value of geriatric assessments, biomarkers reflecting biological age and sarcopenia with respect to discontinuation rate, toxicity and efficacy
- To assess Quality of Life
- To analyze the value of polymorphism of genes involved in drug metabolism in predicting bortezomib-induced PNP
- To assess the prognostic value of risk factors at diagnosis, including β2-microglobulin and chromosomal abnormalities
- To analyze the prognostic value of myeloma gene expression profiles
- Cost effectiveness analysis
Eligibility
- Inclusion Criteria:
- Previously untreated patients with a confirmed diagnosis of symptomatic multiple myeloma according to IMWG criteria (see appendix A)
- Age ≥ 75 years
- WHO performance status 0-3, WHO 4 performance status is allowed when related to MM (see appendix E)
- Measurable disease as defined by the presence of M-protein in serum or urine and/or abnormal free light chain (FLC) ratio with involved FLC (see appendix A for definitions). (If plasmacytoma is the only measurable parameter, the patient is not allowed to be included in the study, because of difficult response evaluation).
- Patient gives consent for extra bone marrow, blood and skin biopsy sampling
- Written informed consent.
- Exclusion Criteria:
- Non-secretory MM
- Systemic Amyloid Light-chain (AL) amyloidosis
- Polyneuropathy, grade 1 with pain or grade ≥ 2
- Severe cardiac dysfunction (NYHA classification IV, appendix F)
- Severe pulmonary dysfunction defined as breathlessness at rest
- Significant hepatic dysfunction (total bilirubin ≥ 30 μmol/l or transaminases ≥ 3 times normal level), unless related to MM
- Renal insufficiency requiring dialysis
- Patients with active, uncontrolled infections
- Pre-treatment with cytostatic drug, immunomodulatory drugs (IMiDs) or proteasome inhibitors. Radiotherapy or a short course of steroids (e.g. 4 day treatment of dexamethasone 40 mg/day or equivalent) are allowed
- Patients known to be Human Immunodeficiency Virus (HIV)-positive
- Active malignancy other than MM requiring treatment or a malignancy that has been treated with chemotherapy currently affecting bone marrow capacity
- Any psychological, familial, sociological and geographical condition potentially hampering compliance with the study protocol and follow-up schedule
- Patients with plasma cell leukemia
Registration Details
Eligible patients should be registered before start of treatment. Patients need to be registered at the HOVON Data Center by one of the following options:
- By ALEA; Use goto eCRF button > select the [Patient tab] and click the [Add new patient] button. Complete all items and click the [Submit] button
- By faxing the completed registration/randomization CRF +31 (0)10 704 1028 Monday through Friday, from 09:00 to 17:00 CET
- By phone +31 (0)10 704 1560 Monday through Friday, from 09:00 to 17:00 CET
The following information will be requested at registration:
- Protocol number
- Institution name
- Name of caller/responsible investigator
- Sex
- Date of birth
- Date written informed consent
- Specific items patient gives consent for (see ICF)
- Eligibility criteria
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
