HOVON HO126 MM
Main info
- Identificatie:
- HO126 MM
- Sponsor:
- HOVON
- Working group party:
- Myeloma
- Age:
- >= 18
- Stadium:
- 1st lijn
- Echelon:
- Level D
- Included patients:
-
147(of 144)
- Active sites:
-
40(of 40)
- Title:
Ixazomib citrate-thalidomide-low dose dexamethasone induction followed by maintenance therapy with ixazomib citrate or placebo in newly diagnosed multiple myeloma patients not eligible for autologous stem cell transplantation; a randomized phase II trial.
Timeline
News
21-04-2022: The final study analysis of the HOVON126 took place.
e-CRFs/ALEA can no longer be updated, query answers will no longer be processed.
You will soon receive the final study documents and the instructions on how to proceed with your HOVON126 Investigator Site File.
Flow
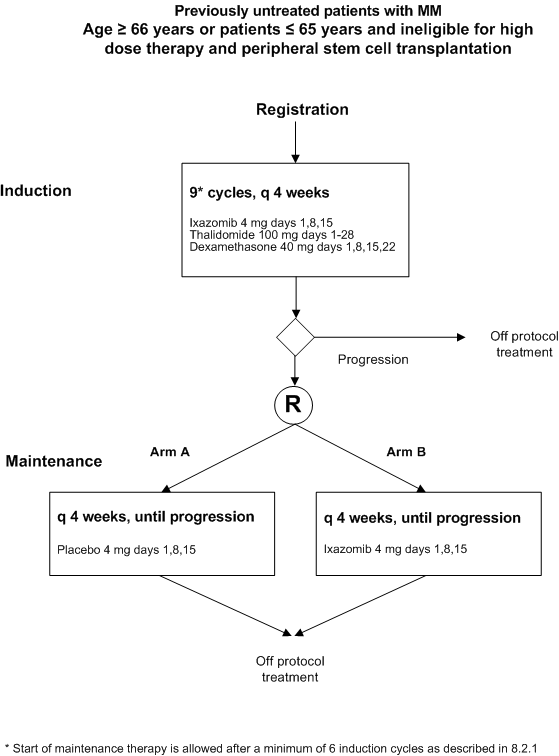
Details
- Phase:
- Prospective Phase II study
- Monitoring Type:
- Not any more
- Objectives:
'Primary objectives
- Maintenance treatment
- To compare progression free survival between maintenance therapy with Ixazomib versus placebo, both following induction therapy with ixazomib citrate – thalidomide – low dose dexamethasone
- To determine overall response rate of induction therapy with ixazomib citrate – thalidomide – low dose dexamethasone. Overall response will be defined as (stringent) complete response, very good partial response and partial response (protocol appendix C).
Secondary objectives
- To determine toxicity, polyneuropathy in specific, during induction treatment and maintenance treatment with ixazomib citrate or placebo
- To determine progression free survival and overall survival from registration
- To compare the efficacy between maintenance treatment with ixazomib citrate versus placebo determined as overall survival from randomization
- To determine the efficacy of maintenance therapy determined as an improvement in response during maintenance treatment with ixazomib citrate or placebo
- To determine efficacy of induction therapy determined as time to response
- To determine feasibility, defined as discontinuation rate due to toxicity, during induction treatment and maintenance treatment with ixazomib citrate or placebo
- To determine time to next treatment
- To determine PFS on second line therapy
- To determine Quality of Life during induction treatment and maintenance therapy with Ixazomib or placebo
- To identify clinical, imaging-related and molecular markers prognostic and predictive for outcome and toxicity
- To establish second primary malignancies (SPM)'
- Maintenance treatment
Eligibility
- Inclusion Criteria:
'* Previously untreated patients with a confirmed diagnosis of symptomatic multiple myeloma according to IMWG criteria (see protocol appendix A)
- Measurable disease according to the IMWG criteria (see protocol appendix A). (If plasmacytoma is the only measurable parameter, the patient is not allowed to be included in the study, because of difficult response evaluation)
- Age ≥ 66 years or patients ≤ 65 years not eligible for ASCT
- WHO performance status 0-3 for patients <75 years and WHO performance status 0-2 for patients ≥ 75 years (see protocol appendix D)
- Absolute neutrophil count (ANC) ≥ 1.0 x109/l and platelet count ≥ 75x109/l , unless related to bone marrow infiltration by malignant plasmacells. Platelet transfusions to help patients meet eligibility criteria are not allowed within 3 days before study enrollment
- Written informed consent
- Patient gives consent for extra bone marrow and blood sampling
- Negative pregnancy test at study entry or at least 1 year post-menopausal or surgically sterile before study entry
- A female patient of childbearing potential, agrees to practice 2 effective methods of contraception, at the same time, from the time of signing the informed consent through 90 days after the last dose of study drug, AND must also adhere to the guidelines of any treatment-specific pregnancy prevention program (for thalidomide) OR agrees to completely abstain from heterosexual intercourse. (Periodic abstinence (eg, calendar, ovulation, symptothermal, post-ovulation methods) and withdrawal are not acceptable methods of contraception)
- Male patients, even if surgically sterilized, (i.e., status post vasectomy) must agree to practice effective barrier contraception during the entire study period and through 90 days after the last dose of study drug, AND must also adhere to the guidelines of any treatment-specific pregnancy prevention program (for thalidomide), OR agrees to completely abstain from heterosexual intercourse (Periodic abstinence (eg, calendar, ovulation, symptothermal, post-ovulation methods] and withdrawal are not acceptable methods of contraception).'
- Exclusion Criteria:
'* Known allergy to any of the study medications, their analogues, or excipients in the various formulations of any agent
- Systemic AL amyloidosis
- Polyneuropathy, grade 3 or higher or grade 2 with pain on clinical examination during the screening period
- Evidence of current uncontrolled cardiovascular conditions, including uncontrolled hypertension, uncontrolled cardiac arrhythmias, symptomatic congestive heart failure, unstable angina, or myocardial infarction within the past 6 months
- Severe pulmonary dysfunction (Modified Medical Research Counsil dyspnea scale classification III-IV)
- Significant hepatic dysfunction (total bilirubin ≥ 1.5 x ULN or transaminases ≥ 3 times normal level) except patients with Gilbert’s syndrome as defined by > 80% unconjugated bilirubin
- Creatinine clearance <30 ml/min or Calculated Glomerular Filtration Rate [ml/min/1.73m2] <30.
- Systemic treatment, within 14 days before the first dose of ixazomib, with strong CYP3A inducers (rifampin, rifapentine, rifabutin, carbamazepine, phenytoin, phenobarbital), or use of Ginkgo biloba or St. John’s wort. Pre-treatment with cytostatic drug, IMIDs or proteasome inhibitors. Radiotherapy or a short course of steroids (e.g. 4 day treatment of dexamethasone 40 mg/day or equivalent) are allowed. Radiotherapy should not be given within 14 days before enrollment. In case of palliative radiotherapy for pain control and if the involved field is small, 7 days will be considered a sufficient interval between treatment and administration of the ixazomib citrate
- Not able and/or not willing to use adequate contraception
- Female patients who are lactating or have a positive serum pregnancy test during the screening period
- Major surgery within 14 days before enrollment
- Central nervous system involvement
- Ongoing or active systemic infection, active hepatitis B or C virus infection, or known human immunodeficiency virus (HIV) positive
- Known GI disease or GI procedure that could interfere with the oral absorption or tolerance of ixazomib citrate including difficulty swallowing
- Diagnosed or treated for another malignancy within 2 years before study enrollment or previously diagnosed with another malignancy and have any evidence of residual disease. Patients with nonmelanoma skin cancer or carcinoma in situ of any type are not excluded if they have undergone complete resection
- Participation in other clinical trials, including those with other investigational agents not included in this trial, within 21 days of the start of this trial and throughout the duration of this trial
- Any serious medical or psychiatric illness, or familial, sociological and geographical condition potentially hampering compliance with the study protocol and follow-up schedule'
Registration Details
'Eligible patients should be registered before start of treatment. Patients need to be registered at the HOVON Data Center by one of the following options:
- Trial Online Process (TOP, https://www.hdc.hovon.nl/top). A logon to TOP can be requested at the HOVON Data Center for participants.
- By faxing the completed registration/randomization CRF +31.10.7041028 Monday through Friday, from 09:00 to 17:00 CET
- By phone +31.10.7041560 Monday through Friday, from 09:00 to 17:00 CET'
Follow Up.
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
