HOVON HO146 ALL
Main info
- Identificatie:
- HO146 ALL
- Sponsor:
- HOVON
- Working group party:
- Leukemia
- Age:
- 18-70
- Stadium:
- 1st lijn
- Echelon:
- Level B
- Included patients:
-
71(of 71)
- Active sites:
-
15(of 18)
- Title:
Blinatumomab added to prephase and consolidation therapy in precursor B-acute lymphoblastic leukemia in adults.
Timeline
News
- 01-12-2020: The study is closed. No more patients can be registered.
- Use the following link to go to the HO146 app: https://srv130.fr/hovon146/
Number of patients to be included in the HO146: 1 (27 nov.)
Important: Only patients who are on the 'HO146 screening list' (i.e., patient with a 'SCR146- letter code hospital-XXX' number is assigned) are eligible to be actually registered by us in ALEA. Patient registration in ALEA currently takes place exclusively via the HOVON Data Center (HDC)
- 23-07-2020: adjustment in lab manual NL & B, E new versions available on website.
- 02-06-2020: adjustment of the Central NL&BE shipping form, new version v2 available on the website
- 20-12-2019: From today (20 Dec 2019) you can register new HO146 patients directly via ALEA. It is therefore no longer possible to register via TOP. ALEA registration patient instruction can be found under 'K CRF guidelines' on the website at section Downloads.
- 11-12-2019: New version RegRand form v2_25NOV2019 placed on the website
- 15-10-2019: New version labmanual v04 for Belgium placed on the website, send samples via SDX
- 08-10-2019: New version labmanual v03 for Belgium placed on the website
- The HOVON 146 study has been approved for the Dutch sites by the CCMO and the central Ethics Committee of the Erasmus MC. This means that you can start the local procedures.
Flow
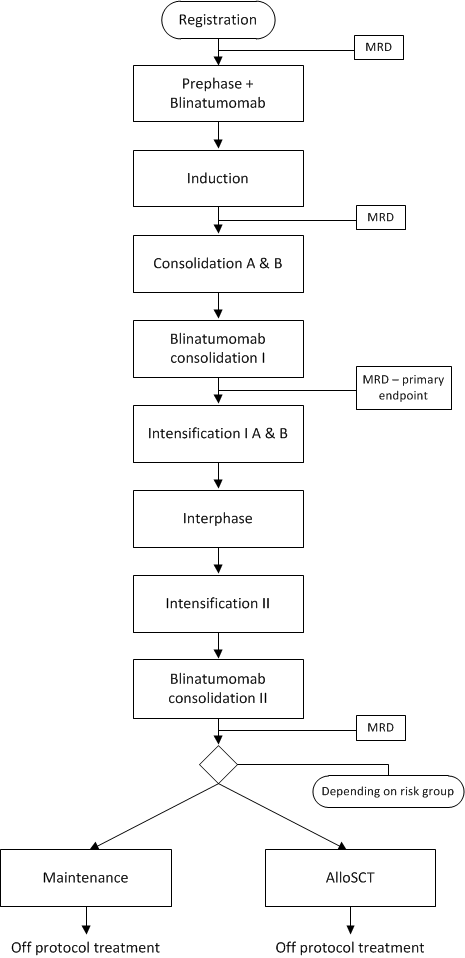
Details
- Phase:
- Prospective Phase II study
- Monitoring Type:
- Site Evaluation Visit
- Objectives:
Primary objective:
- To assess the proportion of patients that achieve MRD negative response (by PCR) after the first consolidation phase including blinatumomab
Secondary objectives:
To assess the MRD level following induction chemotherapy- To assess the MRD level after second blinatumomab consolidation
- To assess the hematological response after induction, blinatumomab consolidation I and blinatumomab consolidation II
- To evaluate event-free survival (EFS)
- To evaluate relapse-free survival (RFS)
- To evaluate overall survival (OS)
- To evaluate safety and toxicity of blinatumomab
- To assess clinical outcome of patients receiving maintenance or allogeneic SCT
- To assess kinetics of T-cells and B-cells and their various subsets during treatment and assess their predictive value as regard to molecular response
- To compare the results of molecular and flowcytometric MRD measurements at the same timepoints.
Eligibility
- Inclusion Criteria:
- Primary CD19 positive precursor B-ALL (excluding mature B-cell ALL and B-lymphoblastic lymphoma, but including Philadelphia positive/BCR-ABL positive ALL) and CD19 positive mixed phenotype acute lymphoblastic leukemia (MPAL);
- Patients aged 18 to 70 years inclusive;
- WHO performance status 0-2;
- Negative pregnancy test at inclusion, if applicable;
- Written informed consent;
- Patient is capable of giving informed consent.
- Exclusion Criteria:
- Mature B-cell leukemia/lymphoma, B-lymphoblastic lymphoma, isolated extramedullary disease;
- CML in blast crisis;
- Acute undifferentiated leukemia;
o Basal or squamous cell carcinoma of the skin
o Carcinoma in situ of the cervix
o Carcinoma in situ of the breast
o Incidental histologic finding of prostate carcinoma- Patient known to be HIV-positive;
- Pregnant or breast-feeding female patients;
- Unwilling or not capable to use effective means of birth control (all men, all premenopausal women under the age of 50 need contraception for two years after the last period, and women older than 50 years for at least one year);
- Current participation in another clinical trial;
- Any psychological, familial, sociological and geographical condition potentially hampering compliance with the study protocol and follow-up schedule.
Registration Details
Eligible patients should be registered before start of treatment of Blinatumomab. Patients need to be registered at the HOVON Data Center by one of the following options:
- ALEA (https://aleaclinical.com/Hovon/DM/DELogin.aspx?refererPath=DEHome.aspx). A logon to ALEA can be requested at the HOVON Data Center for participants
- By faxing the completed registration/randomization CRF +31.10.7041028 Monday through Friday, from 09:00 to 17:00 CET
- By phone +31.10.7041560 Monday through Friday, from 09:00 to 17:00 CET
Planned HOVON interim/final analyses for the coming 6 months: Interim analysis (ASH-2020; Q2-2020)
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
