HOVON HO151 NHL
Main info
- Identificatie:
- HOVON 151 DLBCL
- Sponsor:
- HOVON
- Working group party:
- Lymphoma
- Age:
- 18-75
- Stadium:
- 1st lijn
- Echelon:
- Level C-HIC&C-SCT
- Included patients:
-
109(of 109)
- Active sites:
-
31(of 30)
- Title:
A phase II study evaluating the feasibility and clinical efficacy of atezolizumab consolidation treatment in high risk diffuse large B-cell lymphoma
Timeline
News
09SEP2024: Updated CTR Protocol v5 (27FEB2024) is now available and addendum 2 ICF for the Netherlands (archiving for 25 years instead of 15 years)
Flow
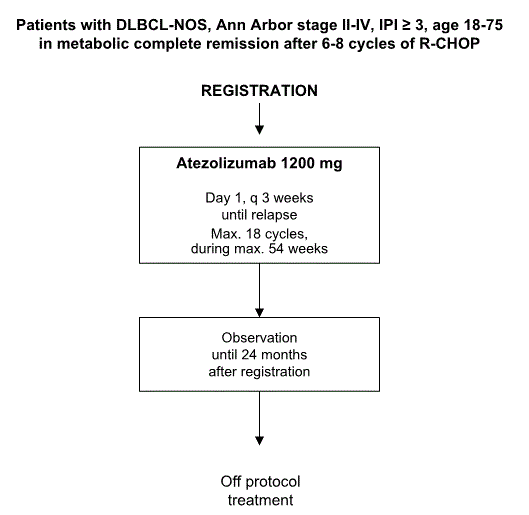
Details
- Phase:
- Prospective Phase II study
- Monitoring Type:
- Not any more
- Objectives:
Primary objective
- To evaluate the 2-year DFS for patients in complete metabolic remission after R-CHOP induction.
Note: The 2-year event free survival, defined as relapse, re-treatment or death, was established as a robust end point for disease related outcome in DLBCL.81 Since in this study re-treatment can only be initiated after relapse, 2-years EFS equals 2-years DFS
Secondary objective
- To evaluate toxicity and assess the relation of adverse events in time to recovery of the Tcell repertoire.
- To evaluate the 2-year OS.
- To evaluate MRD status at the end of induction therapy, at various time points during consolidation treatment and at the end of consolidation (see paragraph 10.5.1).
- To evaluate the recovery of the T-cell and NK cell repertoire after induction therapy and at various time points during consolidation treatment in relation to toxicity and efficacy (see paragraph 10.5.3)
Exploratory objectives
- To explore the PDL1/HLA expression, mutational load, gene expression immune profile, soluble PDL1, microbiome and T-cell clonality of patients in relation to MRD status and MRD conversion. (see paragraph 10.5.2).
- To explore the tumor characteristics and mutational dynamics in patients who relapse (see paragraph 10.5.6).
- To assess the crossing of the blood-brain barrier of atezolizumab by measuring atezolizumab concentrations in het cerebrospinal fluid.
Eligibility
- Inclusion Criteria:
- Age 18-75 (inclusive) years.
- Patients with a confirmed histologic diagnosis of diffuse large B-cell lymphoma (DLBCL-NOS) based upon a representative histology specimen according to the WHO classification, revision 2016 (see appendix A).
- Ann Arbor stages II-IV (see appendix B).
- WHO performance status 0 – 1 (see appendix E).
- IPI ≥ 3 at diagnosis (see appendix C).
- Complete metabolic remission (Deauville 1-3) after 6-8 cycles of R-CHOP according to the Lugano criteria (see appendix D).
- Of note:
- Rituximab may have been administered either intravenously or subcutaneously. A rituximab biosimilar may have been used when it is approved for the indication of DLBCL.
- Only dose reductions for vincristine are allowed during R-CHOP. Patients should have received a cumulative dose that equals at least 6 cycles R-CHOP without dose reduction.
- Central nervous system prophylaxis (MTX) by intrathecal or IV therapy is allowed.
- F-FDG-PET scan should have been made 4-8 weeks after last induction cycle.
- Negative pregnancy test at study entry.
- Patient is willing and able use adequate contraception during and until 5 months after the last protocol treatment.
- Written informed consent
- Patient is capable of giving a written informed consent.
- Exclusion Criteria:
Diagnosis
- All histopathological diagnoses other than DLBCL-NOS according to the WHO classification, revision 2016 (see appendix A), including:
- High-grade B-cell lymphoma with a double/triple translocation with MYC, BCL2 and/or BCL6. Please note that patients with an isolated MYC translocation or an isolated BCL2 translocation or an isolated BCL-6 translocation are eligible (single hit translocation)
- Testicular large B-cell lymphoma
- Primary mediastinal B cell lymphoma
- Transformed indolent lymphoma
- Post-transplant lymphoproliferative disorder.
Organ dysfunction
- Clinical signs of severe pulmonary dysfunction.
- Clinical signs of heart failure (NYHA classification II-IV).
- Symptomatic coronary artery disease or cardiac arrhythmias not well controlled with medication.
- Myocardial infarction during the last 6 months.
- Significant renal dysfunction (serum creatinine ≥ 150 umol/l or clearance ≤ 30ml/min
- Creatinine clearance may be calculated by Cockcroft –Gault formula: CrCl = (140 - age [in years]) x weight [kg] (x 0.85 for females)/ (0.815 x serum creatinine [μmol/L])
- Inadequate hematological function: hemoglobin < 5.5 mmol/L ANC < 1.0x10^9/L or platelets <75x10^9 /L.
- Spontaneous INR > 1.5, aPTT >33.
- Significant hepatic dysfunction (total bilirubin ≥ 1.5x upper limit of normal (ULN) or transaminases ≥ 2.5 x ULN), unless related to Gilberts syndrome.
- Clinical signs of severe cerebral dysfunction.
- Patients with a history of uncontrolled seizures, central nervous system disorders or psychiatric disability judged by the investigator to be clinically significant and adversely affecting compliance to study drugs.
- Major surgery within the last 4 weeks.
- Known or suspected infection
- Known active bacterial, viral, fungal, mycobacterial, parasitic, or other infection or any major episode of infection requiring treatment with IV antibiotics or hospitalization within 4 weeks before date of registration. Suspected active or latent tuberculosis needs to be confirmed by positive interferon gamma (IFN-γ) release assay.
- Patients known to be HIV-positive.
- Active chronic hepatitis B or C infection.
- Administration of a live, attenuated vaccine within 4 weeks before date of registration or anticipation that such a live attenuated vaccine will be required during the study and for a period of 5 months after discontinuation of atezolizumab.
Auto-immune
- Any active or history of documented autoimmune disease, including but not limited to myasthenia gravis, myositis, autoimmune hepatitis, systemic lupus erythematosus, rheumatoid arthritis, inflammatory bowel disease, vascular thrombosis associated with antiphospholipid syndrome, Wegener’s granulomatosis, Sjögren’s syndrome, Guillain-Barré syndrome, multiple sclerosis, vasculitis, or glomerulonephritis. The following exceptions are allowed: Patients with autoimmune-related hypothyroidism or type 1 diabetes mellitus who are on stable treatment.
- History of idiopathic pulmonary fibrosis, organizing pneumonia (e.g., bronchiolitis obliterans), drug-induced pneumonitis, idiopathic pneumonitis, or evidence of active pneumonitis per chest CT scan at screening.
- Patients with uncontrolled asthma or allergy, requiring systemic steroid treatment.
- Regular treatment with corticosteroids within the 4 weeks prior to date of registration, unless administered for indications other than NHL at a dose equivalent to < 30 mg/day prednisone/prednisolone.
General
- Serious underlying medical conditions, which could impair the ability of the patient to participate in the trial (e.g. ongoing infection, uncontrolled diabetes mellitus, gastric ulcers, active autoimmune disease)
- Current participation in another clinical trial interfering with this trial
- History of active cancer during the past 5 years, except basal cell carcinoma of the skin or stage 0 cervical carcinoma
- Life expectancy <6 months
- Any psychological, familial, sociological and geographical condition potentially hampering compliance with the study protocol and follow-up schedule
Prior treatment
- Prior treatment with atezolizumab, or anti PD-1 or PDL-1 antibodies.
- Prior treatment with CD137 agonists or immune checkpoint blockade therapies, including anti-CTLA4 therapeutic antibodies.
- Treatment with systemic immunostimulatory agents (including but not limited to IFN, interleukin [IL]-2) within 6 weeks or 5 half-lives of the drug, whichever is shorter, prior to date of registration.
- Treatment with systemic immunosuppressive medications, including but not limited to prednisone, cyclophosphamide, azathioprine, methotrexate, thalidomide, and anti-tumor necrosis factor (anti-TNF) agents within 2 weeks prior to date of registration; inhaled corticosteroids and mineralocorticoids are allowed.
- All histopathological diagnoses other than DLBCL-NOS according to the WHO classification, revision 2016 (see appendix A), including:
Registration Details
Eligible patients should be registered before start of treatment. Patients need to be registered at the HOVON Data Center by one of the following options:
- By ALEA; select the [patient] tab and click the [ Add new patient] button. Complete all items and click the [Submit] button
- By faxing the completed registration/randomization CRF +31.10.7041028 Monday through Friday, from 09:00 to 17:00 CET
- By phone +31.10.7041560 Monday through Friday, from 09:00 to 17:00 CET
Primary endpoint analysis (Q2-2024)
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
