HOVON HO157 AML
Geannuleerd
Main info
- Identificatie:
- HO157
- Sponsor:
- HOVON
- Working group party:
- Leukemia
- Age:
- >= 18
- Stadium:
- 1st lijn
- Echelon:
- Limited Site Selection
- Included patients:
-
0(of 140)
- Active sites:
-
15(of 40)
- Title:
A randomized phase II multicenter study to assess the feasibility and efficacy of the addition of an interphase cycle with 10-day decitabine prior to start conditioning for an allogeneic HCT in AML and high risk MDS (IPSS-R > 4.5) with detectable MRD after at least 2 cycles of intensive chemotherapy
Timeline
Scheduled
Actual
2019
21 mei
Opportunity
2019
28 nov.
Cancelled
News
29AUG2019; Glycomimetics has withdrawn its support to the study, the study is therefore cancelled.
Flow
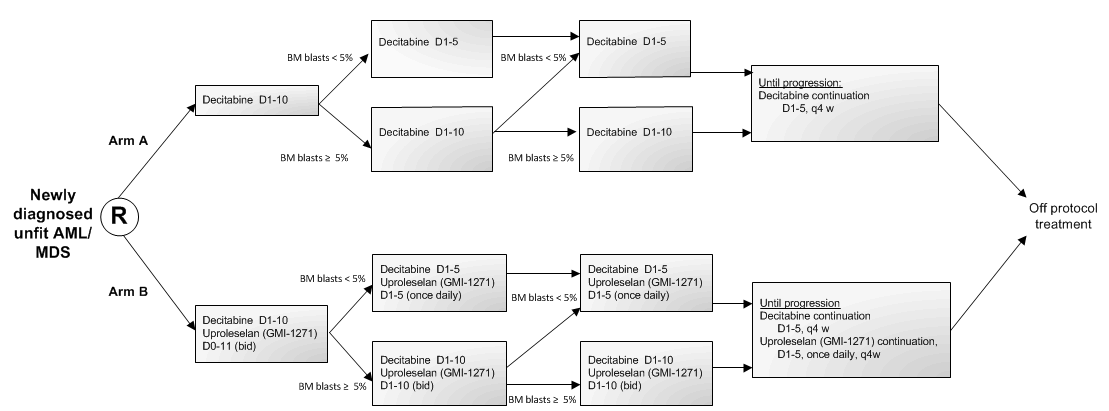
Details
- Phase:
- Prospective randomized Phase II study
- Monitoring Type:
- Study Specific
- Objectives:
Primary objectives:
- To assess in a randomized comparison the effect of uproleselan (GMI-1271) added to 10-day decitabine treatment on the cumulative CR/CRi rate during 3 cycles.
Secondary objectives:
- To assess the safety and tolerability of uproleselan (GMI-1271) added to 10-day decitabine treatment for AML (frequency and severity of toxicities and the durations of neutropenia and thrombocytopenia) as regards to the selected dose level of the study. Further, specifically, the number of events of fever, sepsis and pneumonia will be assessed in both arms. In addition, the all cause 60-day mortality will be assessed in both arms.
- To determine the efficacy profile: response rate (CRMRD-, CR, CRi, MLFS, PR), event free survival (EFS) and overall survival (OS) associated with the two therapy regimens.
- To measure MRD by immunophenotyping and PCR in relation to clinical response parameters.
- To identify gene mutations and E-selectin ligand expression on leukemic blasts as potential biomarkers predictive of response, EFS and OS by exploratory analysis.
- To evaluate the prognostic value of baseline physical and functional conditions using comprehensive geriatric assessment tools (short physical performance battery (SPPB) and activities of daily living (ADL)) on treatment outcome.
Eligibility
- Inclusion Criteria:
- Patients with:
- a diagnosis of AML and related precursor neoplasms according to WHO 2016 classification (excluding acute promyelocytic leukemia) including secondary AML (after an antecedent hematological disease (e.g. MDS) and therapy-related AML, or
- a diagnosis of myelodysplastic syndrome with excess of blasts (MDS) and IPSS-R > 4.5
- Patients 18 years and older.
- Patients NOT eligible for standard chemotherapy, defined as HCT-CI ≥ 3 or Patients NOT eligible for standard chemotherapy for other reasons (wish of patient).
- WBC ≤ 30 x10^9/L (prior hydroxyurea allowed for a maximum of 5 days, stop 2 days before start decitabine treatment)
- Adequate renal and hepatic functions unless clearly disease related as indicated by the following laboratory values:
- Serum creatinine ≤ 2.5 mg/dL (≤ 221.7 µmol/L), unless considered AML-related
- Serum bilirubin ≤ 2.5 x upper limit of normal (ULN), unless considered AML-related or due to Gilbert’s syndrome
- Alanine transaminase (ALT) ≤ 2.5 x ULN, unless considered AML-related
- WHO performance status 0, 1 or 2
- Male patients and female patients (if indicated) must use an effective contraceptive method during the study and for a minimum of 3 months after study treatment.
- Written informed consent.
- Patient is capable of giving informed consent.
- Patients with:
- Exclusion Criteria:
- Acute promyelocytic leukemia.
- Acute leukemia's of ambiguous lineage according to WHO 2016
- Patient has symptomatic central nervous system (CNS) leukemia (NO routinely lumbar puncture required to investigate CNS involvement)
- Blast crisis of chronic myeloid leukemia.
- Diagnosis of any previous or concomitant malignancy is an exclusion criterion:
- except when the patient completed successfully treatment (chemotherapy and/or surgery and/or radiotherapy) with curative intent for this malignancy at least 6 months prior to randomization OR
- except for basal and squamous cell carcinoma of the skin or in situ carcinoma of the cervix
- Patients previously treated for AML (any antileukemic therapy including investigational agents), a short treatment period ( ≤ 5 days) with Hydroxyurea is allowed
- Current concomitant chemotherapy, radiation therapy, or immunotherapy; other than hydroxyurea
- Concurrent severe and/or uncontrolled medical condition (e.g. uncontrolled diabetes, infection, hypertension, pulmonary disease etc.)
- Cardiac dysfunction as defined by:
- Myocardial infarction within the last 3 months of study entry, or
- Reduced left ventricular function with an ejection fraction < 40% as measured by MUGA scan or echocardiogram or
- Unstable angina or
- New York Heart Association (NYHA) grade IV congestive heart failure (see Appendix I) or
- Unstable cardiac arrhythmias
- History of stroke or intracranial hemorrhage within 6 months prior to randomization.
- Patient has a history of human immunodeficiency virus (HIV) or active infection with Hepatitis C or B.
- Patients known to be pregnant
- Patients with a history of non-compliance to medical regimens or who are considered unreliable with respect to compliance.
- Patients with any serious concomitant medical condition which could, in the opinion of the investigator, compromise participation in the study.
- Patients who have senile dementia, mental impairment or any other psychiatric disorder that prohibits the patient from understanding and giving informed consent.
- Any psychological, familial, sociological or geographical condition potentially hampering compliance with the study protocol and follow-up schedule
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
Site
15 results
Order by
Accrual rate
Activation date
NL-Amersfoort-MEANDERMC
LT-Vilnius-SANTA
BE-Roeselare-AZDELTA
BE-Haine-Saint-Paul-JOLIMONT
BE-Antwerpen-ZNASTUIVENBERG
NL-Zwolle-ISALA
NL-Rotterdam-ERASMUSMC
NL-Maastricht-MUMC
NL-Leeuwarden-MCL
NL-Groningen-UMCG
NL-Enschede-MST
NL-Breda-AMPHIA
NL-Amsterdam-VUMC
NL-Amsterdam-OLVG
LU-Luxembourg-CHL
