HOVON HO176 NHL
Main info
- Identificatie:
- HOVON 176 DLBCL
- Sponsor:
- HOVON
- Working group party:
- Lymphoma
- Stadium:
- 2de lijn
- Echelon:
- Level C-HIC&C-SCT
- Included patients:
-
13
- Active sites:
-
6(of 10)4 sites are pending
- Title:
Golcadomide (CC-99282) for relapsed and refractory large B-cell lymphoma of the central nervous system: a phase 2 study
Timeline
Flow
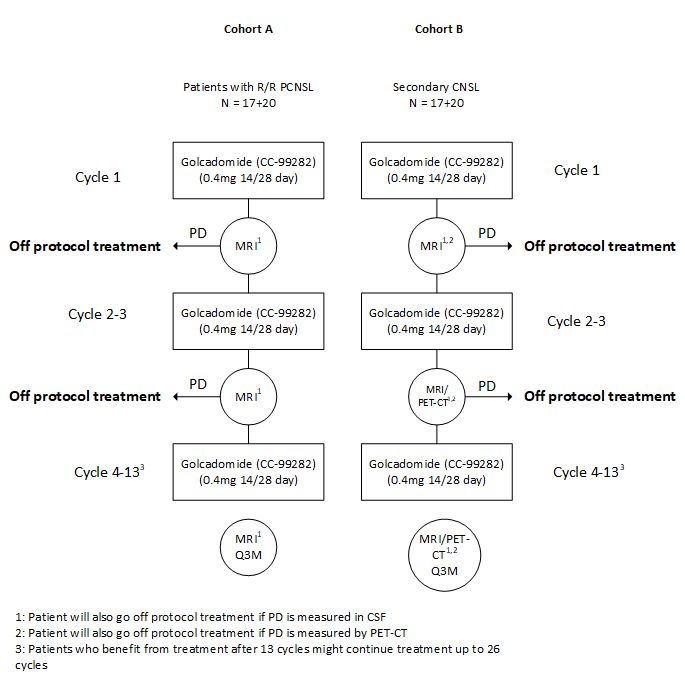
Details
- Phase:
- Prospective Phase II study
- Monitoring Type:
- HOVON Monitoring Visit
- Objectives:
Primary objective:
- The primary objective of the study is a best observed ORR (complete remission (CR) and partial remission (PR)) of 40%, according to the IPCG response criteria among patients with R/R PCNSL and according to International PCNSL Collaborative Group (IPCG) and Lugano criteria among patients with sCNSL.
Secondary objectives:
- To determine toxicity according to the Common Terminology Criteria for Adverse Events (CTCAE) grading (Appendix I)
- To evaluate time to best response
- To evaluate PFS as measured from time of start study treatment until progression or death.
- To evaluate OS as measured from time of start study treatment until death of any cause.
- To evaluate DOR as measured from first documentation of response until relapse or progression or death. To assess functional status and quality of life (QoL).
Eligibility
- Inclusion Criteria:
In order to be eligible to participate in this study, a patient must meet all of the following criteria:
Cohort A and cohort B:
- ≥ 18 years as minimum age.
- World Health Organization (WHO) performance status ≤ 2
- Patient must understand and voluntarily sign an Informed Consent Form (ICF) prior to any study related assessments/procedures being conducted.
- Patient is willing and able to adhere to the study visit schedule and other protocol requirements.
- Haemoglobin > 5 mmol/l.
- Absolute neutrophil count (ANC) >1.0x10^9/l without growth factor support for 7 days.
- Platelet count >75x10^9/l without transfusions for 7 days.
- Patient agrees not to participate in any other interventional study while on protocol treatment without approval of the Principal Investigator.
Additional inclusion criteria cohort A
- Diagnosis of a R/R PCNSL according to the WHO 2022 classification.
- Patients must have received prior high-dose methotrexate based chemotherapy.
Additional inclusion criteria cohort B
- Diagnosis of aggressive malignant B-cell lymphoma based upon a representative histology specimen according to the WHO 2022 classification.
- Follicular lymphoma (FL) grade 3B or transformed FL.
- DLBCL
- HGBCL with MYC and BCL2 rearrangements.
- Progression or relapse with CNS localization with or without systemic relapse.
- Diagnosis of CNS localization at inclusion based on at least one of the following:
- Unequivocal morphological and/or immunophenotypically evidence of Cerebrospinal Fluid (CSF) lymphoma.
- Clinical AND Magnetic resonance imaging (MRI) evidence of leptomeningeal localization.
- Brain parenchymal lesion showing homogeneous contrast enhancement suspect for lymphoma, concurrently with systemic progression or recurrence.
- Biopsy-proven brain parenchymal NHL localization of previously diagnosed systemic NHL.
- Exclusion Criteria:
A patient who meets any of the following criteria cannot be included in this study:
Cohort A and cohort B:
- The following DLBCL subtypes are not allowed:
- Patients with intravitreal lymphoma.
- Primary testicular lymphoma.
- Intravascular lymphoma.
- Epstein-Barr virus (EBV) driven lymphoma.
- Post-transplant LPDs.
- Patient has received prior therapy with CRBN-modulating drug (e.g., lenalidomide, avadomide/CC-122, pomalidomide, golcadomide) within 5 half-lives or 4 weeks, whichever is shorter, prior to starting investigational product (IP).
- Severe cardiovascular disease (arrhythmias not well controlled with medication, congestive heart failure or symptomatic ischemic heart disease).
- Severe pulmonary dysfunction.
- Severe neurological or psychiatric disease.
- Significant hepatic dysfunction (serum transaminases ≥ 3 times x upper limit of normal (ULN) or bilirubin ≥ 3 x ULN (unless bilirubin rise is due to Gilbert's syndrome)).
- Significant renal dysfunction (estimated creatinine clearance < 45 ml/min after rehydration according to local practice).
- Concurrent severe and/or uncontrolled medical condition (e.g. uncontrolled diabetes, infection, hypertension, cancer, etc.).
- Active or uncontrolled viral infection (Hepatitis B Virus (HBV), Hepatitis C Virus (HCV), Human Immunodeficiency Virus (HIV), Coronavirus disease (COVID)).
- History of active malignancy (other than lymphoma) requiring ongoing treatment during the past 5 years with the exception of basal carcinoma of the skin or stage 0 cervical carcinoma.
- Patient is pregnant, breast-feeding patients, or intending to become pregnant during participation in the study.
- Any psychological, familial, sociological and geographical condition potentially hampering compliance with the study protocol and follow-up schedule.
- Hypersensitivity to the active substance.
- Patients unable to swallow capsules or with diseases significantly affecting the gastrointestinal function..
Registration Details
Eligible patients should be registered before start of treatment. Patients need to be registered at HOVON via the registration database/eCRF.
Most recent link to the eCRF is on top of the HOVON website.
The following information will be requested at registration:
- Protocol number
- Institution name
- Name of caller/responsible investigator
- Sex
- Age at registration
- Date written informed consent
- Specific items patient gives consent for (see ICF)
- Eligibility criteria
- Stratification factors (either R/R PCNSL or sCNSL)
All eligibility criteria will be checked with a checklist. Each patient will be given a unique patient study number (a sequence number by order of enrolment in the trial). Patient study number will be given immediately by the online registration database or by phone and confirmed by email.
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
