HOVON HO18 ALL
Gearchiveerd
Main info
- Identificatie:
- HO18 ALL
- Sponsor:
- HOVON
- Age:
- Any age
- Stadium:
- 1st lijn
- Included patients:
-
210
- Active sites:
-
0(of 17)17 sites are pending
- Title:
Autologous Bone Marrow Transplantation with a Radiation-free Preparatory Regimen (Bu/Cy) in adult patients with ALL in the first remission after Conventional Induction and Intensification Chemotherapy: A Phase II Study.
Timeline
Scheduled
Actual
1993
15 jun.
Activated
1999
05 apr.
Closeout in Progress
2009
05 apr.
Archived
2025
15 jun.
Destruction
Flow
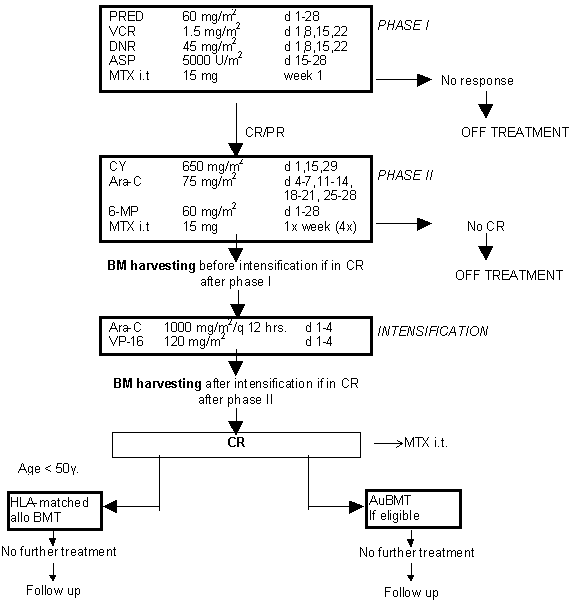
Details
- Phase:
- Prospective Phase II study
- Monitoring Type:
- Objectives:
Eligibility
- Inclusion Criteria:
- Age between 16 and 60 years.
- Previously untreated.
- All according to the FAB criteria.
- AUL according to immunological marker analysis.
- Patients must have persistant serum creatinine < 200 �mol/l.
- Patients must have serum direct bilirubin < 65 �mol/l.
- WHO performance status grade 0, 1, 2 or 3.
- Absence of other severe disease.
- Exclusion Criteria:
- Performance status grade 4 according to the WHO classification.
- Severe cardiac, pulmonary, hepatic, renal, neurologic, psychiatric or metabolic disease.
- Second primary malignant disease, except cervix carcinoma and non-melanoma skin cancer.
- Persisting renal insufficiency.
- Patients previously treated with chemotherapy.
- Active uncontrolled infections.
- HIV positivity on serological tests.
Registration Details
Central registration at the HOVON Data Center:
Erasmus MC Cancer Institute, Clinical Trial Center (Hs-423)
P.O. Box 2040
NL-3000 CA Rotterdam
Phone number.: +31.10.7041560 (working days 9.00-17.00)
Fax number.....: +31.10.7041028
TOP address...: http://www.hdc.hovon.nl/top
Registration criteria
The following information will be requested:
- Protocol number
- Institutions name
- Patients namecode
- Date of birth
- Hospital record number
- Name of responsible investigator
- Date of start treatment
Participating Sites
Site
17 results
Order by
Accrual rate
Activation date
NL-Amsterdam-VUMC
35
NL-Utrecht-UMCUTRECHT
28
NL-Rotterdam-ERASMUSMC
25
BE-Leuven-UZLEUVEN
22
NL-Rotterdam-EMCDANIEL
21
NL-Maastricht-MUMC
20
NL-Amsterdam-AMC
11
NL-Den Haag-HAGA
11
NL-Enschede-MST
7
NL-Sittard-Geleen-ZUYDERLAND
7
NL-Zwolle-ISALA
6
NL-Amersfoort-MEANDERMC
4
NL-Heerlen-ATRIUMMC
4
NL-Nieuwegein-ANTONIUS
3
NL-Amsterdam-SLOTERVAART
1
NL-Leeuwarden-MCL
1
NL-Utrecht-DIAKONESSENUTRECHT
1
