HOVON HO32 MDS
Gearchiveerd
Main info
- Identificatie:
- HO32 MDS / ELDERLY AML
- Sponsor:
- HOVON
- Included patients:
-
137
- Active sites:
-
0(of 16)16 sites are pending
- Title:
Randomised Phase II Study on the Value of Fludarabine in addition to ARA-C and G-CSF in the Treatment of Patients with Myelodysplastic Syndromes and Elderly Acute Myeloid Leukemia.
Timeline
Scheduled
Actual
1996
14 okt.
Activated
2006
14 okt.
Archived
2021
14 okt.
Destruction
2021
14 okt.
Destruction
Flow
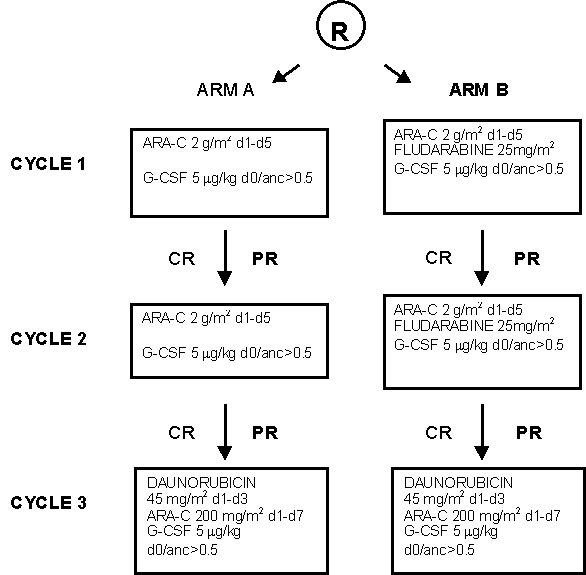
Details
- Phase:
- Prospective randomized Phase II study
- Monitoring Type:
- Objectives:
Eligibility
- Inclusion Criteria:
- Patients with ages between 15 and 75 years with a primary or secondary hematological diagnosis of myelodysplasia.
- Patients with ages between 61 and 75 years with a primary or secondary diagnosis of AML.
- Performance status 0, 1 or 2 according to the WHO performance scale
- Serum creatinin < 120 �mol/l
- Serum bilirubin < 25 �mol/l. ALAT and ASAT and AP less than 2.5 times the upper limit of normal.
- Informed consent.
- Exclusion Criteria:
- Severe cardiac, pulmonary, neurologic, metabolic or psychiatric disease.
- Malignancy, except stage I cervix carcinoma and basal carcinoma of the skin.
- Active uncontrolled infections.
- Chemotherapy within 6 months before entry to the study.
- Pregnancy.
- Blastic crisis of CML, or leukemia developing from Mypro.
Participating Sites
Site
16 results
Order by
Accrual rate
Activation date
BE-Leuven-UZLEUVEN
28
NL-Amsterdam-VUMC
23
NL-Rotterdam-EMCDANIEL
18
NL-Rotterdam-ERASMUSMC
14
BE-Brugge-AZBRUGGE
12
NL-Nieuwegein-ANTONIUS
10
NL-Utrecht-UMCUTRECHT
8
NL-Enschede-MST
4
NL-Groningen-UMCG
4
NL-Nijmegen-RADBOUDUMC
4
BE-Roeselare-AZDELTA
3
NL-Amsterdam-SLOTERVAART
2
NL-Amsterdam-AMC
2
NL-Zwolle-ISALA
2
NL-Amersfoort-MEANDERMC
2
NL-Zutphen-GELREZUTPHEN
1
