HOVON HO34 MDS
Gearchiveerd
Main info
- Identificatie:
- HO34 MDS / EORTC 06961
- Sponsor:
- HOVON
- Active sites:
-
0(of 1)1 sites are pending
- Title:
Autologous Peripheral Blood Stem Cell Transplantation (PSCT) versus a second Intensive Consolidation Course after a Common Induction and Consolidation Course in Patients with bad prognosis Myelodysplatic Syndromes (MDS) and Acute Myelogenous Leukemia secondary (SAML) to MDS of more than 6 months duration: A Phase III Study.
Timeline
Scheduled
Actual
1996
15 dec.
Activated
2006
15 dec.
Archived
2010
27 mei
Closeout in Progress
2025
27 mei
Destruction
Flow
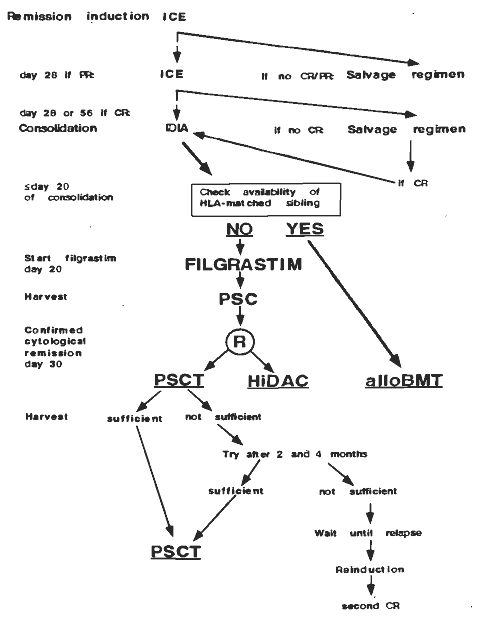
Details
- Phase:
- Prospective randomized Phase III study
- Monitoring Type:
- Objectives:
Eligibility
- Inclusion Criteria:
- All patients with untreated RAEBt, RAEB with > 10% blast cells in the bone marrow, other forms of MDS with multiple chromosomal abnormalities, and/or profound cytopenias, and secondary AML supervening after overt MDS of more than 6 months duration are eligible for the study.
- Patients between 10 and 60 years.
- Informed consent and expected cooperation of the patient with regard to treatment and follow-up.
- Performance status according to WHO: scale 0, 1 or 2.
- Exclusion Criteria:
- Blast crisis of chronic myeloid leukemia.
- Leukemias supervening after other myeloproliferative diseases.
- Leukemias supervening after overt MDS of less than 6 months duration.
- Inadequate renal and liver function.
- Patients with severe heart failure.
- Patients with severe concomitant neurological disease.
- Other progressive malignant diseases. However, secondary acute leukemias following cured Hodgkin�s disease or other malignancies will be included, as well as secondary leukemias following other exposure to alkylating agents or radiations for any other reason.
- Patients already treated for their MDS or AML by intensive chemotherapy, and/or radiotherapy.
- Patients treated with biological response modifiers and/or low dose Ara-C within 4 weeks prior to entry.
Participating Sites
Informatie is niet langer beschikbaar.
Site
1 results
Order by
Accrual rate
Activation date
HOVON Data Center
