HOVON HO41
Gearchiveerd
Main info
- Identificatie:
- HOVON 41 AL Amyloidosis
- Sponsor:
- HOVON
- Included patients:
-
70
- Active sites:
-
13(of 13)
- Title:
Autologous Stem Cell Transplantation for Patients with AL Amyloidosis: A Prospective Phase II Study.
Timeline
Scheduled
Actual
2002
04 sep.
Activated
2006
31 jan.
Closeout in Progress
2015
13 okt.
Archived
2030
13 okt.
Destruction
Flow
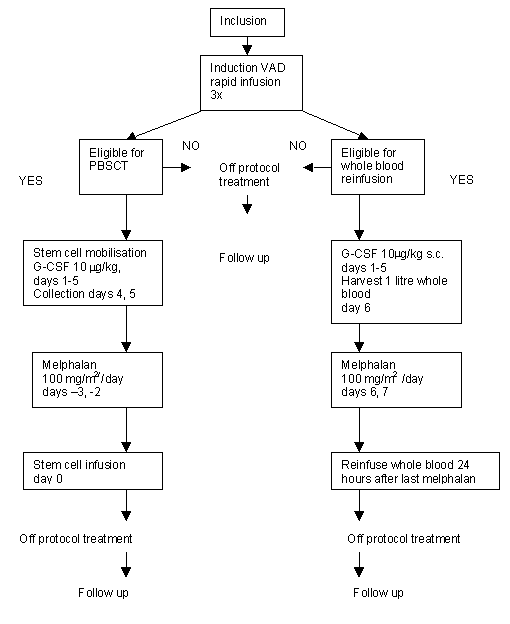
Details
- Phase:
- Prospective Phase II study
- Monitoring Type:
- Objectives:
Eligibility
- Inclusion Criteria:
- Age under 66 years
- MGUS, multiple myeloma stage I
- Histologically documented systemic AL amyloidosis (see appendix C)
- Untreated or previously treated with maximal 3 courses of melphalan and prednisone
- The patient must give informed consent according to the rules of the hospital.
- Exclusion Criteria:
- Prior malignancies diagnosed less than 5 years ago, except non-melanoma skin tumours or stage 0 (in situ) cervical carcinoma. Note: Prior malignancies diagnosed and treated longer than 5 years ago, and in which case it is likely that the patient is cured, are no exclusion criteria.
- Patients with familial variants of systemic amyloidosis.
- Severe pulmonary, neurologic, psychiatric, cardiac, liver or metabolic disease not related to AL amyloidosis.
Participating Sites
Site
13 results
Order by
Accrual rate
Activation date
NL-Utrecht-UMCUTRECHT
11
NL-Amsterdam-VUMC
10
NL-Maastricht-MUMC
9
NL-Rotterdam-ERASMUSMC
8
NL-Nijmegen-RADBOUDUMC
8
BE-Leuven-UZLEUVEN
8
NL-Groningen-UMCG
7
NL-Den Haag-HAGA
4
NL-Leiden-LUMC
2
NL-Amsterdam-AMC
2
NL-Nieuwegein-ANTONIUS
1
NL-Amersfoort-MEANDERMC
NL-Apeldoorn-GELREAPELDOORN
