HOVON HO44
Gearchiveerd
Main info
- Identificatie:
- HOVON 44 NHL
- Sponsor:
- HOVON
- Included patients:
-
239
- Active sites:
-
25(of 25)
- Title:
A randomized phase III study on the effect of the chimeric anti-CD20 monoclonal antibody (MabThera) during sequential chemotherapy followed by autologous stem cell transplantation in patients with relapsed or progressive B-cell non-Hodgkin's lymphoma.
Timeline
Scheduled
Actual
2000
20 nov.
Activated
2005
23 dec.
ClosedForInclusionActualStart
2005
23 dec.
Closeout in Progress
2015
23 dec.
Archived
2030
23 dec.
Destruction
Flow
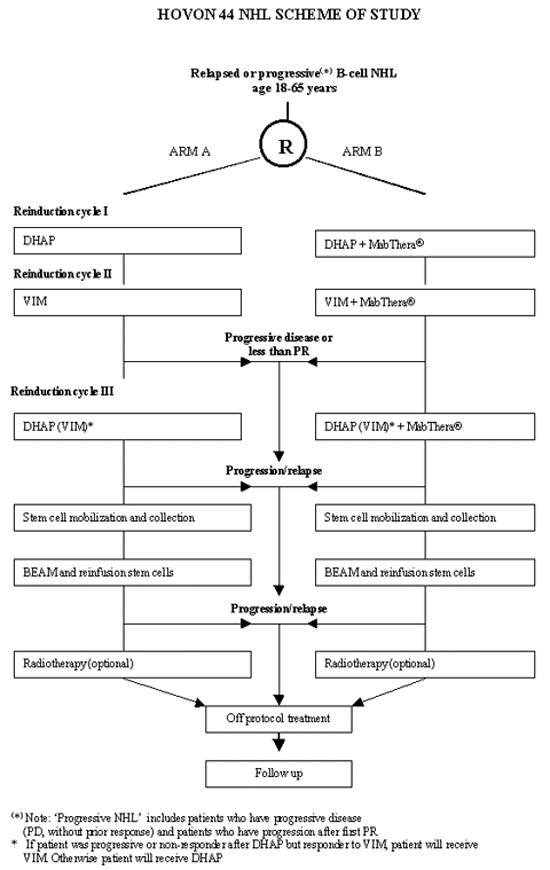
Details
- Phase:
- Prospective randomized Phase III study
- Monitoring Type:
- Objectives:
Eligibility
- Inclusion Criteria:
- Malignant lymphoma based upon a representative histology specimen according to the REAL classification at relapse or progression: Follicular center lymphoma, follicular (grade III), Diffuse large B-cell lymphoma, Primary mediastinal B-cell lymphoma
- CD20 positive
- First progression or relapse during/after adriamycin containing regimen. *'Progressive' includes patients who have progressive disease (PD, without prior response) and patients who have progression after first PR
- Age 18-65 years inclusive
- WHO performance status 0 - 1 (see appendix E)
- Witnessed written informed consent according to the center requirements
- Exclusion Criteria:
- Patients with history of intolerance of exogenous protein administration
- Patients with severe cardiac dysfunction (NYHA classification II-IV, appendix F)
- Patients with severe pulmonary dysfunction (vital capacity or diffusion capacity < 70% of predicted value) unless clearly related to NHL involvement
- Patients with hepatic dysfunction, bilirubin or transaminase >= 2.5 x upper normal limit
- Patients with renal dysfunction (serum creatinine >=180 mumol/l or clearance <=40 ml/min)
- Prior treatment with immunotherapy or radiation therapy within the last month before entering the study
- Patients with active uncontrolled infections
- Patients known to be HIV-positive
- Patients with NHL localization in the central nervous system
- Patients with (EBV) post-transplant lymphoproliferative disorder
Participating Sites
Site
25 results
Order by
Accrual rate
Activation date
NL-Groningen-UMCG
45
NL-Amsterdam-VUMC
36
NL-Rotterdam-ERASMUSMC
27
NL-Rotterdam-EMCDANIEL
21
NL-Amsterdam-AMC
18
NL-Leiden-LUMC
16
NL-Den Haag-HAGA
15
NL-Utrecht-UMCUTRECHT
11
NL-Amersfoort-MEANDERMC
9
NL-Amsterdam-AVL
7
NL-Amsterdam-OLVG
6
NL-Dordrecht-ASZ
6
NL-Nieuwegein-ANTONIUS
6
NL-Enschede-MST
6
NL-Zwolle-ISALA
2
NL-Roosendaal-BRAVIS
2
NL-Utrecht-DIAKONESSENUTRECHT
2
NL-Amsterdam-SLOTERVAART
1
BE-Leuven-UZLEUVEN
1
NL-Den Haag-HMCBRONOVO
1
NL-Den Haag-HMCWESTEINDE
1
NL-Deventer-DZ
NL-Delft-RDGG
NL-Dirksland-VANWEELBETHESDA
NL-Leidschendam-HMCANTONIUSHOVE
