HOVON HO49
Gearchiveerd
Main info
- Identificatie:
- HOVON 49 MM
- Sponsor:
- HOVON
- Included patients:
-
344
- Active sites:
-
0(of 53)53 sites are pending
- Title:
Randomized phase III study in elderly patients with a multiple myeloma on the value of Thalidomide added to Melphalan plus Prednisone.
Timeline
Scheduled
Actual
2002
01 aug.
Activated
2015
16 dec.
Closeout in Progress
2017
15 jul.
Archived
2030
16 dec.
Destruction
Flow
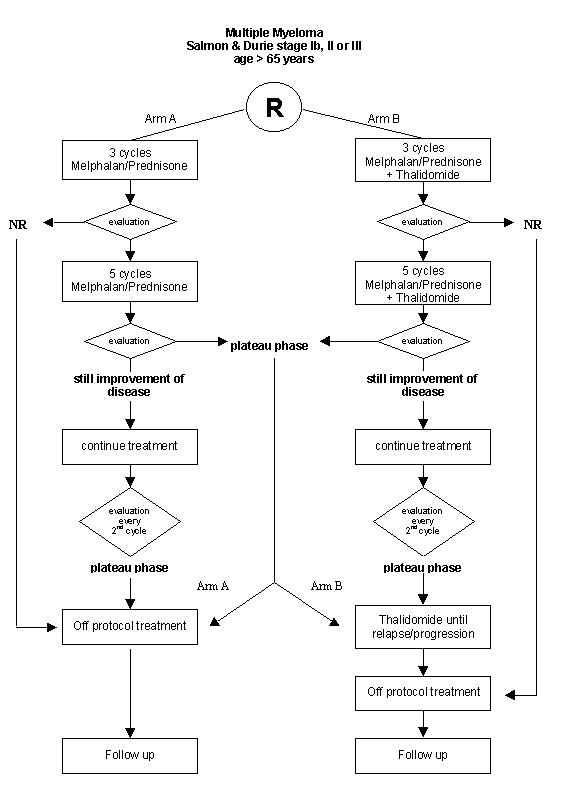
Details
- Phase:
- Prospective randomized Phase III study
- Monitoring Type:
- Objectives:
Eligibility
- Inclusion Criteria:
- Patients with a confirmed diagnosis of multiple myeloma stage Ib, II or III according to the Salmon & Durie criteria (Appendix A)
- Age > 65 years
- WHO performance status 0-3 (see appendix D)
- Measurable tumorparameter (M-protein or Bence Jones prote﮵ria)
- Written informed consent.
- Exclusion Criteria:
- Known intolerance to Thalidomide
- Systemic AL amyloidosis
- Polyneuropathy
- Severe cardiac dysfunction (NYHA classification II-IV, appendix E)
- Severe pulmonary dysfunction
- Significant hepatic dysfunction (total bilirubin >= 30 umol/l or transaminases >= 2.5 times normal level), unless related to myeloma
- Renal failure with dependency on dialysis
- Patients with active, uncontrolled infections
- Pre-treatment with cytostatic drug or alpha interferon
- Patients known to be HIV-positive
- History of active malignancy during the past 5 years, except basal carcinoma of the skin or stage 0 cervical carcinoma
Participating Sites
Site
53 results
Order by
Accrual rate
Activation date
NL-Rotterdam-ERASMUSMC
20
NL-Amersfoort-MEANDERMC
19
NL-Enschede-MST
18
NL-Zwolle-ISALA
13
NL-Amsterdam-VUMC
13
NL-Breda-AMPHIA
13
NL-Utrecht-DIAKONESSENUTRECHT
12
NL-Rotterdam-MAASSTADZIEKENHUIS
10
NL-Nieuwegein-ANTONIUS
10
NL-Heerlen-ATRIUMMC
9
NL-Groningen-UMCG
9
NL-Sittard-Geleen-ZUYDERLAND
9
NL-Meppel-ISALADIACONESSEN
8
NL-Leidschendam-HMCANTONIUSHOVE
8
NL-Maastricht-MUMC
8
NL-Roosendaal-BRAVIS
8
NL-Eindhoven-MAXIMAMC
7
NL-Utrecht-UMCUTRECHT
7
NL-Gouda-GROENEHART
7
NL-Amsterdam-AMC
7
NL-Nijmegen-RADBOUDUMC
6
NL-Venlo-VIECURI
6
NL-Roermond-LZR
6
NL-Dordrecht-ASZ
5
NL-Apeldoorn-GELREAPELDOORN
5
NL-Sneek-ANTONIUSSNEEK
4
NL-Leiden-LUMC
4
NL-Hengelo-ZGTHENGELO
4
NL-Rotterdam-EMCDANIEL
4
NL-Amsterdam-OLVG
4
NL-Delft-RDGG
4
NL-Dirksland-VANWEELBETHESDA
4
NL-Terneuzen-ZORGSAAM
3
NL-Zoetermeer-LANGELAND
3
NL-Rotterdam-SFG
3
NL-Leiderdorp-ALRIJNELEIDERDORP
3
NL-Den Haag-HMCBRONOVO
3
NL-Amstelveen-AMSTELLAND
3
NL-Haarlem-SPAARNEHAARLEM
3
NL-Groningen-MARTINI
2
NL-Leeuwarden-MCL
2
NL-Hoogeveen-TRENTBETHESDA
2
NL-Emmen-SCHEPER
2
NL-Zutphen-GELREZUTPHEN
2
NL-Winterswijk-SKBWINTERSWIJK
2
NL-Goes-ADRZ
2
NL-Den Bosch-JBZ
2
NL-Arnhem-RIJNSTATE
2
NL-Den Haag-HMCWESTEINDE
2
NL-Assen-WZA
1
NL-Amsterdam-SLOTERVAART
1
NL-Delfzijl-OMMELANDERDELFZIJL
1
NL-Schiedam-FRANCISCUSVLIETLAND
1
