HOVON HO50
Gearchiveerd
Main info
- Identificatie:
- HOVON 50 MM
- Sponsor:
- HOVON
- Included patients:
-
556
- Active sites:
-
0(of 42)42 sites are pending
- Title:
A randomized phase III study on the effect of Thalidomide combined with Adriamycin, Dexamethasone (AD) and High Dose Melphalan in patients with multiple myeloma.
Timeline
Scheduled
Actual
2001
27 nov.
Activated
2005
01 jun.
Closeout in Progress
2015
01 jun.
Archived
2020
01 jun.
Destruction
Flow
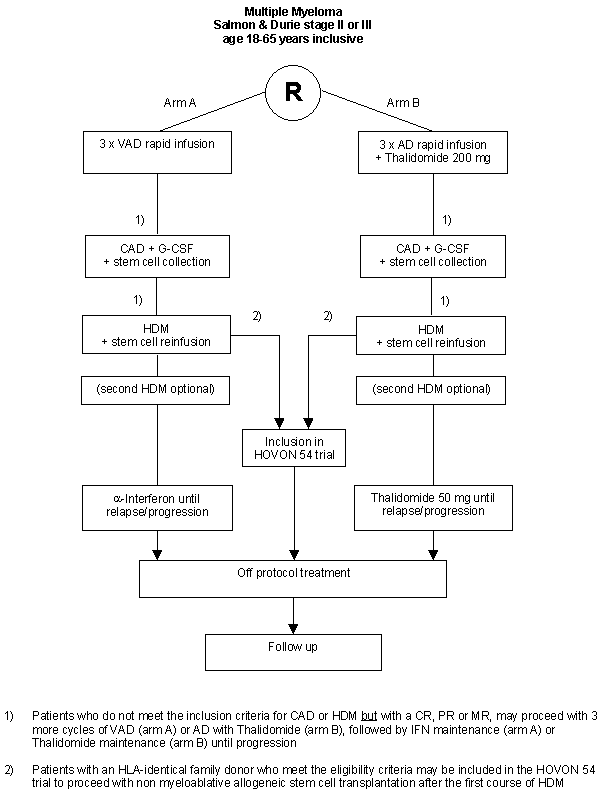
Details
- Phase:
- Prospective randomized Phase III study
- Monitoring Type:
- Objectives:
Eligibility
- Inclusion Criteria:
- Patients with a confirmed diagnosis of multiple myeloma stage II or III according to the Salmon & Durie criteria (see appendix A);
- Age 18-65 years inclusive;
- WHO performance status 0-3 (see appendix D);
- Negative pregnancy test at inclusion if applicable;
- Written informed consent.
- Exclusion Criteria:
- Known intolerance of Thalidomide;
- Systemic AL amyloidosis;
- Previous chemotherapy or radiotherapy except 2 cycles of Melphalan/Prednisone or local radiotherapy in case of local myeloma progression;
- Severe cardiac dysfunction (NYHA classification II-IV, see appendix E) ;
- Significant hepatic dysfunction (serum bilirubin >= 30 mumol/l or transaminases >= 2.5 times normal level), unless related to myeloma;
- Patients known to be HIV-positive;
- Patients with active, uncontrolled infections;
- Patients with a history of active malignancy during the past 5 years with the exception of basal carcinoma of the skin or stage 0 cervical carcinoma;
- Patients who are not willing or capable to use adequate contraception during the therapy (all men, all pre-menopausal women);
- Patients <= 55 years with an HLA-identical sibling who will undergo myeloablative AlloSCT.
Participating Sites
Site
42 results
Order by
Accrual rate
Activation date
NL-Amsterdam-VUMC
60
NL-Rotterdam-ERASMUSMC
54
NL-Groningen-UMCG
45
NL-Nijmegen-RADBOUDUMC
34
NL-Utrecht-UMCUTRECHT
33
NL-Amsterdam-AMC
33
NL-Rotterdam-EMCDANIEL
25
NL-Leiden-LUMC
25
NL-Den Haag-HAGA
23
NL-Nieuwegein-ANTONIUS
19
NL-Enschede-MST
19
NL-Amersfoort-MEANDERMC
17
NL-Den Bosch-JBZ
13
BE-Leuven-UZLEUVEN
13
NL-Zwolle-ISALA
12
NL-Dordrecht-ASZ
12
NL-Maastricht-MUMC
11
NL-Heerlen-ATRIUMMC
11
NL-Rotterdam-SFG
9
NL-Roosendaal-BRAVIS
9
NL-Breda-AMPHIA
9
NL-Sittard-Geleen-ZUYDERLAND
6
NL-Rotterdam-MAASSTADZIEKENHUIS
6
NL-Amsterdam-OLVG
6
NL-Utrecht-DIAKONESSENUTRECHT
5
NL-Hengelo-ZGTHENGELO
5
NL-Apeldoorn-GELREAPELDOORN
5
NL-Zaandam-ZAANSMC
4
NL-Nijmegen-CWZ
4
NL-Arnhem-RIJNSTATE
4
NL-Leeuwarden-MCL
3
NL-Amsterdam-SLOTERVAART
3
NL-Delft-RDGG
2
NL-Gouda-GROENEHART
2
NL-Eindhoven-CATHARINA
2
NL-Haarlem-SPAARNEHAARLEM
2
NL-Eindhoven-MAXIMAMC
2
NL-Leidschendam-HMCANTONIUSHOVE
2
NL-Deventer-DZ
1
NL-Venlo-VIECURI
1
NL-Emmen-SCHEPER
1
NL-Delfzijl-OMMELANDERDELFZIJL
1
