HOVON HO51
Gearchiveerd
Main info
- Identificatie:
- HOVON 51 CML
- Sponsor:
- HOVON
- Included patients:
-
165
- Active sites:
-
15(of 16)
- Title:
A dose-ranging phase I/II study of STI571 in combination with Cytarabin in patients with first chronic phase Chronic Myeloid Leukemia.
Timeline
Scheduled
Actual
2001
14 aug.
Activated
2014
13 okt.
Closeout in Progress
2020
22 okt.
Archived
2029
13 okt.
Destruction
Flow
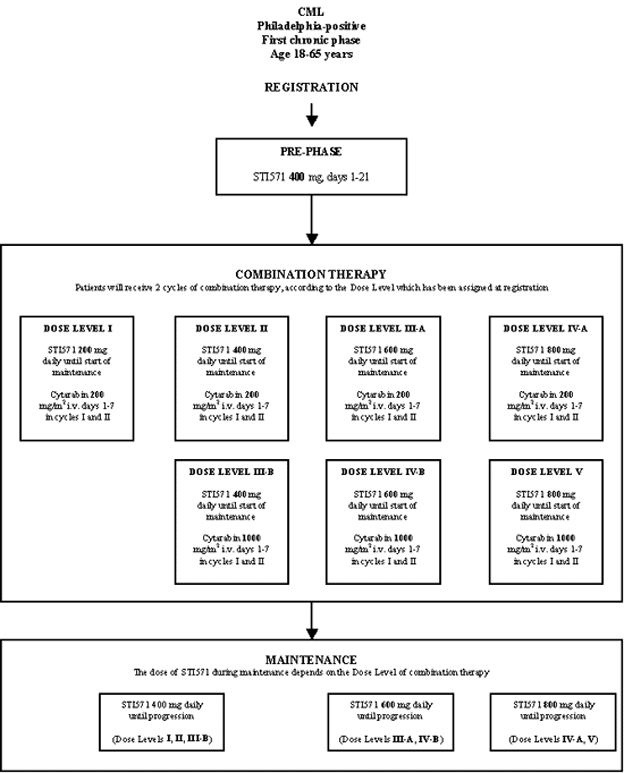
Details
- Phase:
- Prospective Phase I/II study
- Monitoring Type:
- Objectives:
Eligibility
- Inclusion Criteria:
- Newly diagnosed patients with CML in first chronic phase <= 6 months;
- Presence of Philadelphia chromosome or BCR-ABL rearrangement;
- Age 18-65 years inclusive;
- WHO performance status <= 2;
- Written informed consent.
Late randomization
- written informed consent.
- received Imatinib maintenance as HOVON 51 protocol treatment.
- Having achieved a complete molecular response (> 4.5 log reduction by quantitative PCR), which persists for at least 2 years.
- WHO <2
- Exclusion Criteria:
- Hepatic dysfunction (serum bilirubin >= 2 x N, and/or ALAT >= 4 x N);
- Renal dysfunction (creatinin >= 200 mumol/l or 2.3 mg/dl);
- Severe cardiac dysfunction (NYHA classification II-IV, see appendix G) ;
- Severe pulmonary or neurologic disease;
- Pregnant or lactating females;
- Patients with a history of active malignancy during the past 5 years with the exception of basal carcinoma of the skin or stage 0 cervical carcinoma;
- Patients known to be HIV-positive;
- Patients with active, uncontrolled infections;
- Previous treatment other than Hydroxyurea <= 6 months;
Late randomization:
- Unable to visit outpatient clinic at regular one-monthly intervals during the first 6 months after stopping Imatinib.
Participating Sites
Site
15 results
Order by
Accrual rate
Activation date
NL-Rotterdam-EMCDANIEL
37
NL-Amsterdam-VUMC
26
NL-Rotterdam-ERASMUSMC
16
NL-Enschede-MST
14
BE-Leuven-UZLEUVEN
12
NL-Amsterdam-AMC
11
NL-Groningen-UMCG
10
NL-Utrecht-UMCUTRECHT
10
NL-Zwolle-ISALA
6
BE-Bruxelles-STLUC
6
NL-Nijmegen-RADBOUDUMC
6
NL-Amersfoort-MEANDERMC
5
NL-Leiden-LUMC
4
NL-Den Haag-HAGA
2
BE-Brugge-AZBRUGGE
