HOVON HO52
Gearchiveerd
Main info
- Identificatie:
- HOVON 52 AML
- Sponsor:
- HOVON
- Included patients:
-
506
- Active sites:
-
0(of 1)14 sites are pending
- Title:
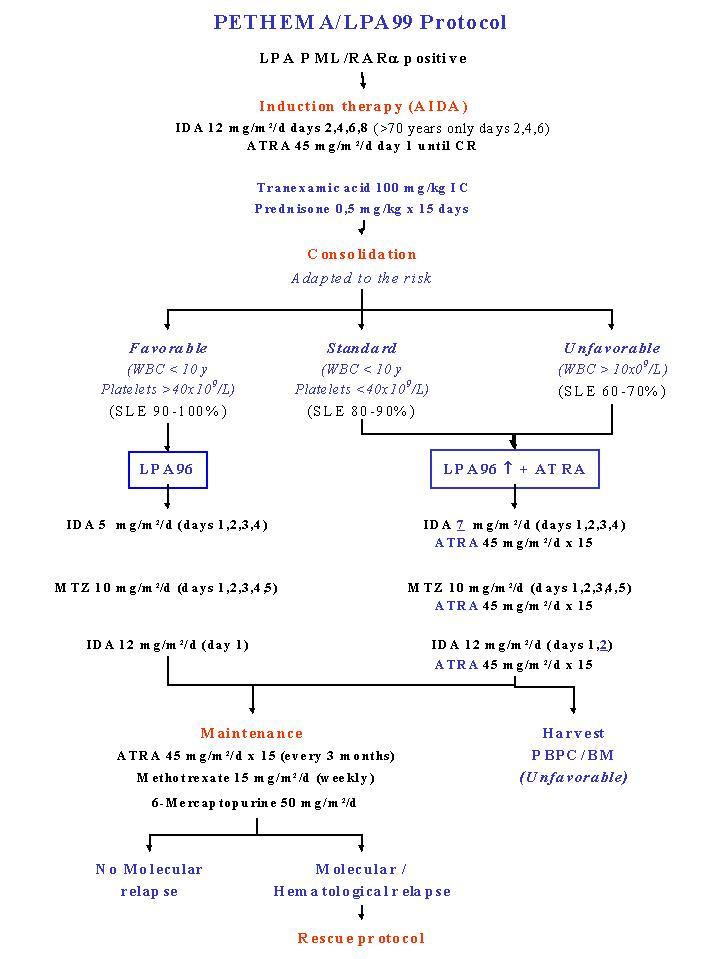
Treatment of Acute Promyelocytic Leukemia: Remission Induction with ATRA+Idarubicin (AIDA). Risk Adapted Intensity of Consolidation and Addition of ATRA Maintenance with ATRA + Methotrexate + Mercaptopurine Rescue Therapy for Molecular and Haematological Relapses.
Timeline
Scheduled
Actual
2001
07 dec.
Activated
2015
13 okt.
Archived
2030
13 okt.
Destruction
Flow
Details
- Phase:
- Prospective Phase II study
- Monitoring Type:
- Objectives:
Eligibility
- Inclusion Criteria:
- Age 18-75 years, inclusive;
- ECOG Performance status <= 3;
- Morphological diagnosis of M3 or M3v. Those cases without typical morphology but with PML-RARa rearrangement may also be included;
- Genetic diagnosis: t(15;17), PML-RARa rearrangement, monoclonal anti-PML positive. Obviously, the result of these tests may become available after having initiated the treatment based on a tentative morphological diagnosis. The presence of secondary cytogenetic changes associated with t(15;17) is not a reason for exclusion nor do they require a different therapeutic approach;
- Written informed consent.
- Exclusion Criteria:
- Age >75 years (the treatment with this protocol can be considered on an individual basis but these patients will be analysed separately);
- Absence of PML-RARa rearrangement;
- Prior antileukemic chemotherapy;
- Presence of an associated neoplasm;
- Presence of a severe psychiatric disease;
- HIV seropositivity;
- Contraindication for intensive chemotherapy, especially to anthracyclines;
- Serum creatinine >= 2.5 mg/dL;
- Bilirubin, alkaline phosphatase, or SGOT > 3 times the upper normal limit;
- Positive pregnancy test;
Participating Sites
Site
14 results
Order by
Accrual rate
Activation date
Non-HOVON-Sites
453
NL-Groningen-UMCG
9
NL-Rotterdam-EMCDANIEL
8
BE-Leuven-UZLEUVEN
8
NL-Rotterdam-ERASMUSMC
8
NL-Amsterdam-AMC
6
NL-Amsterdam-VUMC
4
NL-Den Haag-HAGA
4
NL-Zwolle-ISALA
2
NL-Nieuwegein-ANTONIUS
1
NL-Maastricht-MUMC
1
NL-Enschede-MST
1
NL-Amsterdam-OLVG
1
HOVON Data Center
