HOVON HO63
Gearchiveerd
Main info
- Identificatie:
- HOVON 63 NHL
- Sponsor:
- HOVON
- Included patients:
-
40
- Active sites:
-
31(of 31)
- Title:
Randomized phase III study of Rituximab with intensified CHOP chemotherapy (R-iCHOP-14) versus Rituximab with High-Dose Sequential Therapy and Autologous Stem Cell Transplantation (R-HDT+ASCT) in Adult Patients (18-65 yrs) with Stage II-IV High-intermediate or High Risk Diffuse Large B-Cell Lymphoma.
Timeline
Scheduled
Actual
2005
28 okt.
Activated
2008
25 mrt.
Closeout in Progress
2015
13 okt.
Archived
2030
13 okt.
Destruction
Flow
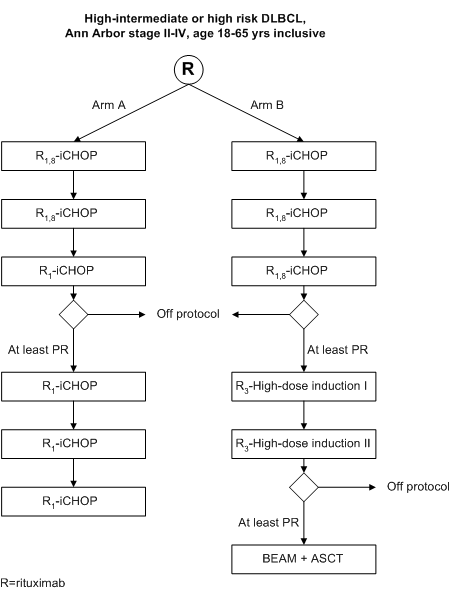
Details
- Phase:
- Prospective randomized Phase III study
- Monitoring Type:
- Objectives:
Eligibility
- Inclusion Criteria:
- Patients with a confirmed histologic diagnosis of DLBCL according to the WHO classification;
- Ann Arbor stage II-IV;
- High-intermediate or high risk NHL according to age-adjusted IPI score (aa IPI=2-3);
- DLBCL must be CD20 positive;
- Age 18-65 years inclusive;
- WHO performance status <= 2;
- Negative preganacy test (if applicable);
- Written informed consent;
- Exclusion Criteria:
- Intolerance of exogenous protein administration;
- Severe cardiac dysfunction (NYHA classification II-IV, Appendix F) or LVEF < 45 %;
- Significant renal dysfunction (serum creatinine >= 150 mmol/l), unless related to NHL;
- Significant hepatic dysfunction (total bilirubin >= 30 mmol/l or transaminases >= 2.5 times normal level), unless related to NHL;
- Suspected or documented Central Nervous System involvement by NHL;
- Testicular DLBCL;
- Primary mediastinal B cell lymphoma;
- Patients known to be HIV-positive;
- Patients with active, uncontrolled infections;
- Patients with uncontrolled asthma or allergy, requiring steroid treatment;
- Patient is a lactating woman;
- Unwillingness or not capable to use effective means of anticonception (all men and pre-menopausal women);
- Prior treatment with chemotherapy, radiotherapy or immunotherapy for this lymphoma, except a short course of prednisone (< 1 wk) and/or cyclophosphamide (< 1 wk and not in excess of 900 mg/m^2 cumulative) or local radiotherapy in order to control life threatening tumor related symptoms;
- History of active cancer during the past 5 years, except basal carcinoma of the skin or stage 0 cervical carcinoma;
Participating Sites
Site
31 results
Order by
Accrual rate
Activation date
NL-Amsterdam-AMC
5
NL-Nieuwegein-ANTONIUS
4
NL-Amsterdam-VUMC
4
NL-Rotterdam-ERASMUSMC
3
NL-Dordrecht-ASZ
3
NL-Delft-RDGG
3
NL-Zwolle-ISALA
2
NL-Rotterdam-EMCDANIEL
2
NL-Leidschendam-HMCANTONIUSHOVE
2
NL-Groningen-UMCG
2
NL-Amersfoort-MEANDERMC
2
NL-Den Haag-HAGA
2
NL-Amsterdam-AVL
1
NL-Rotterdam-MAASSTADZIEKENHUIS
1
NL-Amsterdam-OLVG
1
NL-Den Bosch-JBZ
1
NL-Leeuwarden-MCL
1
NL-Utrecht-UMCUTRECHT
1
NL-Arnhem-RIJNSTATE
NL-Nijmegen-CWZ
NL-Amstelveen-AMSTELLAND
NL-Leiden-LUMC
NL-Sittard-Geleen-ZUYDERLAND
NL-Heerlen-ATRIUMMC
NL-Hoofddorp-SPAARNEGASTHUIS
NL-Enschede-MST
NL-Utrecht-DIAKONESSENUTRECHT
NL-Nijmegen-RADBOUDUMC
NL-Maastricht-MUMC
NL-Den Haag-HMCWESTEINDE
NL-Drachten-NIJSMELLINGHE
