HOVON HO66
Gearchiveerd
Main info
- Identificatie:
- HOVON 66 AML
- Sponsor:
- HOVON
- Active sites:
-
0(of 1)1 sites are pending
- Title:
Hoge dosis ARA-C in combinatie met gemtuzumab ozogamicine (ARGO) als tweedelijns behandeling voor volwassen patienten tot en met 60 jaar met AML en MDS die onvoldoende gereageerd hebben op eerstelijns behandeling.
Timeline
Scheduled
Actual
2020
23 okt.
Archived
Flow
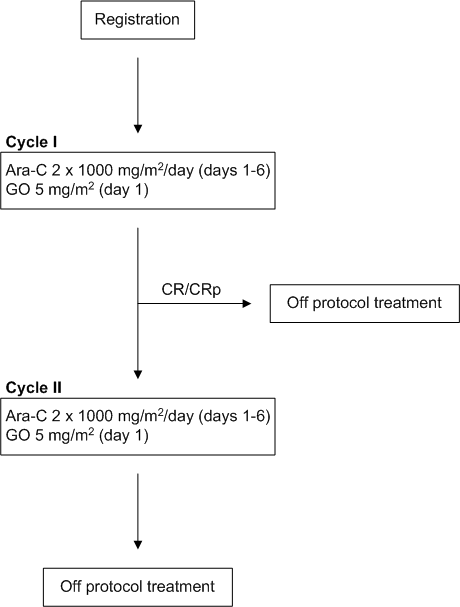
Details
- Phase:
- Prospective Phase II study
- Monitoring Type:
- Objectives:
Eligibility
- Inclusion Criteria:
- Age 18-60 years inclusive;
- Diagnosis (cytopathologically confirmed) of AML (M0-M2 and M4-M7) or RAEB or RAEB-t with an IPSS score of >= 1.5;
- The blood and BM picture, assessed after the first or second remission induction course in the latest non-GO intensive treatment should indicate failure to achieve CR and should be assessed by the local cytologist;
- WHO performance status <= 2;
- Written informed consent;
- Exclusion Criteria:
- AML M3 or AML with cytogenetic abnormality t(15;17) or AML with a PML/RAR alpha or a variant RAR alpha fusion gene;
- Prior stem cell transplant;
- Previous polycythemia rubra vera;
- Primary myelofibrosis;
- Blast crisis of chronic myeloid leukemia;
- Hepatic dysfunction (bilirubin >= 2 x ULN);
- Renal dysfunction (creatinine >= 2 x ULN);
- Concomitant severe lung disease;
- Concomitant severe cardiac dysfunction;
- Concomitant neurological or psychiatric disease;
- Concomitant malignant disease;
- Active uncontrolled infection;
- History of alcohol abuse, i.e. an average of > 5 alcoholic consumptions daily in the preceding year;
- Pregnant or nursing woman;
- Not able or willing to use adequate contraception during therapy (pre-menopausal women);
- Known to HIV positivity;
- Pretreatment with GO;
- Any psychological, familial sociological and geographical condition potentially hampering compliance with the study protocol;
Participating Sites
Informatie is niet langer beschikbaar
Site
1 results
Order by
Accrual rate
Activation date
HOVON Data Center
