HOVON HO67
Gearchiveerd
Main info
- Identificatie:
- HOVON 67 AML SAKK
- Sponsor:
- HOVON
- Included patients:
-
6
- Active sites:
-
9(of 9)
- Title:
Reduced intensity chemotherapy given with and without Imatinib Mesylate in patients >= 60 years considered unfit for standard chemotherapy with previously untreated Acute Myeloid Leukemia (AML) and refractory anemia with excess of Blasts (RAEB, RAEB-t).
Timeline
Scheduled
Actual
2005
16 jun.
Submission in Progress
2005
29 aug.
EC Approval
2006
23 jan.
Activated
2006
15 mrt.
First Site
2006
18 sep.
FPI
2006
01 dec.
ClosedForInclusionActualStart
2020
23 okt.
Archived
2035
23 okt.
Destruction
Flow
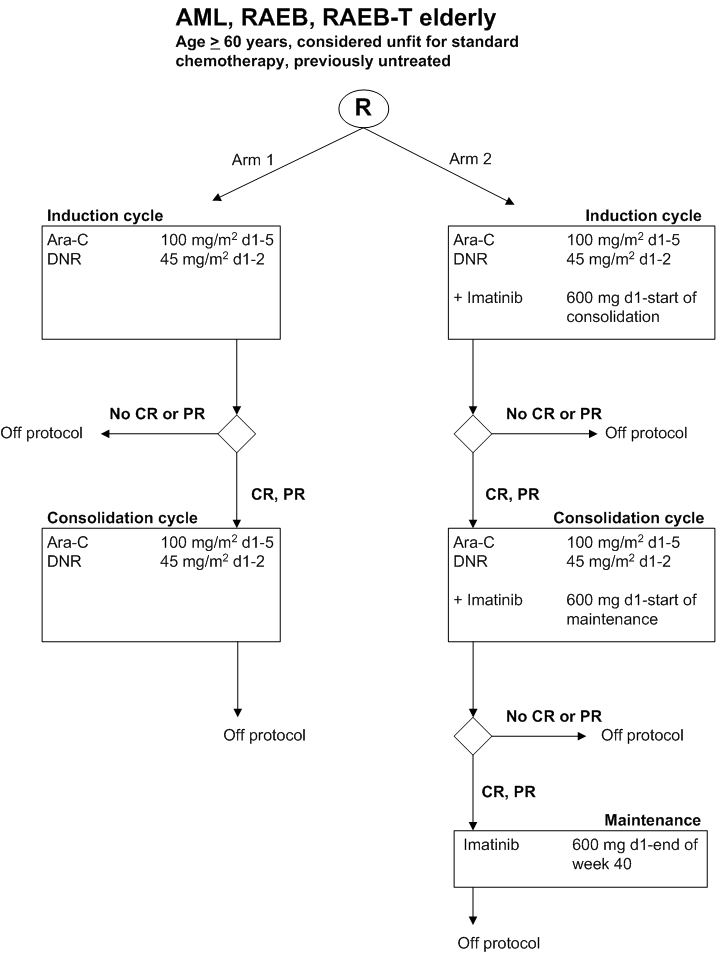
Details
- Phase:
- Prospective randomized Phase II study
- Monitoring Type:
- Objectives:
Eligibility
- Inclusion Criteria:
- Patients >= 60 years.
- Patients considered unfit for standard chemotherapy.
- Patients with a confirmed diagnosis of
a. AML FAB M0-M2 or M4�M7 (see appendix A) or
b. with refractory anemia with excess of blasts (RAEB) or refractory anemia with excess of blasts in transformation (RAEB-T) with an IPSS score > 1.5- Subjects with secondary AML progressing from antecedent (at least 4 months duration) myelodysplasia are also eligible.
- AST (SGOT) and ALT (SGPT), total serum bilirubin, serum creatinine, and creatinine clearance not more than 1.5 x the upper limit of the normal range (ULN) at the laboratory where the analyses were performed.
- Male patients agree to employ an effective barrier method of birth control throughout the study and for up to 3 months following the discontinuation of study drug.
- Written informed consent.
- Exclusion Criteria:
- Patients previously treated for AML (any antileukemic therapy including investigational agents)
- Patients with cardiac dysfunction as defined by:
a. Myocardial infarction within the last 6 months prior to study entry
b. Reduced left ventricular ejection fraction of < 50% as evaluated by echocardiogram or MUGA scan
c. Unstable angina
d. Unstable cardiac arrhythmia- Patients with a history of non-compliance to medical regimens or who are considered potentially unreliable
- Patients with any serious concomitant medical condition, which could, in the opinion of the investigator, compromise participation in the study.
- Patients who have senile dementia, mental impairment or any other psychiatric disorder that prohibits the patient from understanding and giving informed consent.
Participating Sites
Site
9 results
Order by
Accrual rate
Activation date
NL-Rotterdam-EMCDANIEL
2
NL-Den Haag-HAGA
2
NL-Amersfoort-MEANDERMC
1
NL-Rotterdam-ERASMUSMC
1
NL-Amsterdam-AMC
NL-Amsterdam-VUMC
NL-Enschede-MST
NL-Nieuwegein-ANTONIUS
NL-Utrecht-UMCUTRECHT
