HOVON HO81 AML
Main info
- Identificatie:
- HO81 AML
- Sponsor:
- HOVON
- Age:
- > 60
- Stadium:
- 1st lijn
- Echelon:
- Level C-HIC
- Included patients:
-
219(of 33)
- Active sites:
-
34(of 33)
- Title:
A Phase II multicenter study to assess the tolerability and efficacy of the addition of Bevacizumab to standard induction therapy in AML and high risk MDS above 60 years.
Timeline
Flow
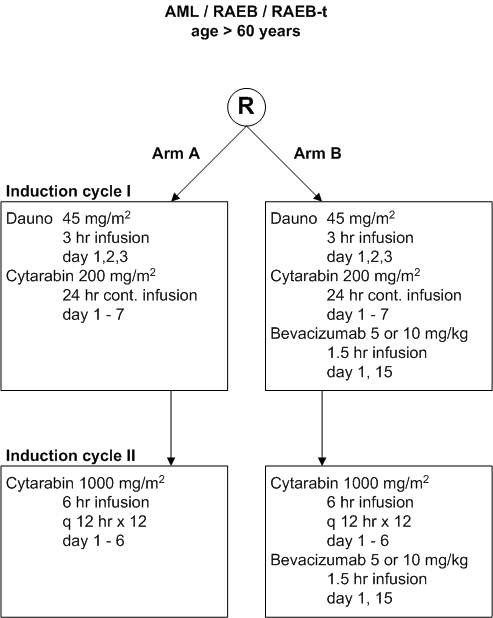
Details
- Phase:
- Prospective Phase II study
- Monitoring Type:
- Not any more
- Objectives:
Primary objectives:
- To assess the safety and tolerability of bevacizumab added to standard induction chemotherapy for AML (frequency and severity of toxicities and the durations of neutropenia and thrombocytopenia)
- To assess in a randomized comparison the effect of bevacizumab on the CR rate.
Secondary objectives:
- To determine the efficacy profile (event free survival and disease free survival) associated with the two therapy regimens.
- To measure MRD by immunophenotyping in relation to clinical response parameters.
- To determine micro vascular density after the first course of therapy in both treatment arms.
- To measure VEGF levels in plasma and VEGF receptors on leukemic blasts in relation to clinical parameters.
In addition, exploratory proteomic and genomic analysis are planned to identify potential biomarkers predictive of response and progression free survival.
Eligibility
- Inclusion Criteria:
- Patients > 60 years.
- Patients eligible for standard chemotherapy.
- Patients with a confirmed diagnosis of
- AML FAB M0-M2 or M4-M7 (see appendix A) or
- with refractory anemia with excess of blasts (RAEB) or refractory anemia with excess of blasts in transformation (RAEB-T) with an IPSS score ≥ 1.5 (see Appendix B)
- Subjects with secondary AML progressing from antecedent (at least 4 months duration) myelodysplasia are also eligible.
- SGOT (AST) and SGPT (ALT) ≤ 1.5 x the upper limit of the normal range (ULN) at the laboratory where the analyses were performed.
- Total serum bilirubin level ≤ 1.5 x the ULN at the laboratory where the analysis was performed.
- Serum creatinine concentration ≤ 1.5 x the ULN at the laboratory where the analysis was performed.
- Proteinuria at baseline: Urine dipstick of proteinuria <2+. Patients discovered to have ≥ 2+ proteinuria on dipstick urinalysis at baseline, should undergo a 24-hour urine collection and must demonstrate ≤ 1 g of protein/24 hr.
- WHO performance status ≤ 2 (see Appendix G)
- Written informed consent.
- Exclusion Criteria:
- Patients previously treated for AML (any antileukemic therapy including investigational agents)
- Past or current history (within the last 2 years prior to randomization) of malignancies except for the indication under this study and curatively treated:
- Basal and squamous cell carcinoma of the skin
- in situ carcinoma of the cervix
- Clinically significant (i.e. active) cardiovascular disease, for example cerebrovascular accidents (≤ 6 months prior to randomization), myocardial infarction (≤ 6 months prior to randomization), unstable angina, New York Heart Association (NYHA) grade II or greater congestive heart failure, serious cardiac arrhythmia requiring medication, reduced left ventricular ejection fraction of < 50% as evaluated by echocardiogram or MUGA scan.
- Uncontrolled hypertension
- Patients with a history of non-compliance to medical regimens or who are considered unreliable with respect to compliance
- Patients with any serious concomitant medical condition which could, in the opinion of the investigator, compromise participation in the study.
- Patients who have senile dementia, mental impairment or any other psychiatric disorder that prohibits the patient from understanding and giving informed consent.
- Major surgical procedure, open biopsy, or significant traumatic injury within 28 days prior to study treatment start, or anticipation of the need for major surgical procedure during the course of the study
- Serious, non-healing wound, ulcer, or bone fracture
- Patients with bleeding diathesis or coagulopathy (unless related to AML)
- Patients with known allergy to Chinese hamster ovary cell proteins or other recombinant human or humanized antibodies or to any excipients of bevacizumab formulation; or to any other study drugs.
Registration Details
Eligible patients who have given written informed consent should be registered and randomised before start of treatment. Patients can be registered and randomised at the HOVON Data Center of the Erasmus MC - Daniel den Hoed by phone call: +31.10.4391568 or fax +31.10.4391028 Monday through Friday, from 09:00 to 17:00, or via the Internet.
The following information will be requested at registration:
- Protocol number
- Institution name
- Name of caller/responsible investigator
- Patients initials or code
- Sex
- Date of birth
- Eligibility criteria
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
