HOVON HO82
Main info
- Identificatie:
- HOVON 82 Sanquin
- Sponsor:
- HOVON
- Included patients:
-
295
- Active sites:
-
8(of 8)
- Title:
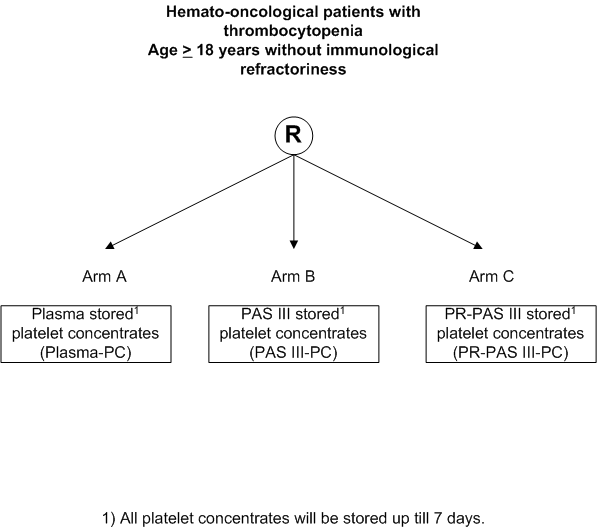
Clinical effectiveness and safety of pooled, random donor platelet concentrates, leucoreduced and stored up to seven days either in additive solution with and without pathogen reduction or plasma in hemato-oncological patients.
Timeline
News
Closure of the HOVON 82 Triplate study
Recently, inclusion in the HOVON 82 study has been stopped after reaching the target number of patients. Earlier, the PRPAS3 (Intercept) arm was closed following the recommendations of the Study Safety Committee. There were no major safety issues, warranting preliminary publication. The final results of the study will be analyzed and presented later this year.
Flow
Details
- Phase:
- Prospective randomized Phase III study
- Monitoring Type:
- Objectives:
- Determination of non-inferiority of PR platelet concentrates
(PR-PAS III-PC) and platelet concentrates in additive solution (PAS III-PC) compared to plasma (plasma-PC), stored for 1-7 days, in terms of recovery, estimated by mean corrected count increment (CCI) at 1 hour.
- To assess the effectiveness in relation to storage time of the used platelet product.
- To evaluate whether clinical factors interact with the different study products leading to a difference in platelet refractoriness.
- To assess the 24 hour CCI.
- To assess the safety (bleeding complications and adverse transfusion reactions).
- To assess the transfusion requirement (red cells and platelets).
- To assess the transfusion interval.
Eligibility
- Inclusion Criteria:
- Age >= 18 years.
- Expected >= 2 platelet transfusion requirements.
- Written informed consent.
- Having a hemato-oncological disease.
- Exclusion Criteria:
- Known immunological refractoriness to platelet transfusions, i.e. HLA- and/or HPA-allo immunization and/or clinical relevant auto-antibodies.
- Pregnancy (or lactating)
- Previous inclusion in this study
