HOVON HO85 NHL
Main info
- Identificatie:
- HOVON 85 NHL
- Sponsor:
- HOVON
- Working group party:
- Lymphoma
- Age:
- >= 18
- Stadium:
- 2de lijn
- Echelon:
- Level C-HIC&C-SCT
- Included patients:
-
64(of 60)
- Active sites:
-
27(of 20)
- Title:
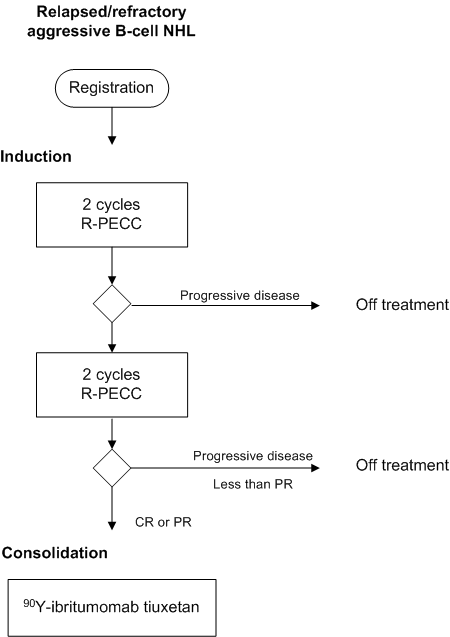
Phase II study on the feasibility and efficacy of consolidation with 90^Y-ibritumomab tiuxetan in patients with relapsed or refractory aggressive B-cell non-Hodgkin’s lymphoma having achieved partial or complete remission after induction with R-PECC chemotherapy.
Timeline
News
End of inclusion: As of March 1, 2012, the HOVON 85 NHL study is closed for entry of new patients since the target number of patients has been reached. We thank you for your cooperation in this study and we hope for a succesfull continuation towards the end of the trial.
New WMO certificate online: The new WMO certificate (which expires APRIL 2013) can now be found on this website.
CRFs: A new version of the CRFs is available. Mainly the R-PECC treatment form has been modified. Please use this new version from now on and discard any old versions.
Flow
Details
- Phase:
- Select:
- Monitoring Type:
- Not any more
- Objectives:
Primary objective:
- Assessment of the feasibility of this treatment approach. Measured primarily by the percentage of patients that reach CR or PR after R-PECC and proceed to 90Y-ibritumomab tiuxetan treatment, and by the fraction of patients that endure the 90Y-ibritumomab tiuxetan treatment without major problems, i.e. the safety and tolerability of 90Y-ibritumomab tiuxetan after R-PECC chemotherapy.
Secondary objectives:
- Efficacy of the 90Y-ibritumomab tiuxetan treatment, measured by the fraction of PR PET positive patients who become PET negative after 90Y-ibritumomab tiuxetan treatment and by the failure free survival and overall survival measured from the start of 90Y-ibritumomab tiuxetan.
- Assessment of the results of the whole treatment approach in terms of the overall response rate, duration of response, failure free survival and overall survival from start of R-PECC treatment.
Eligibility
- Inclusion Criteria:
Inclusion criteria R-PECC induction
- Histologically confirmed aggressive B-cell NHL according to the World Health Organization (WHO) classification (see appendix A):
- Follicular lymphoma grade 3b
- Diffuse large B-cell lymphoma
- Refractory disease or histologically confirmed first or second relapse (Refractory is defined as no response or partial remission according to CT. Patients in partial response (PR) can only be included in case of positive PET scan or positive biopsy)
- CD20 positive (assessed at 1st diagnosis or from fresh histology at confirmation of relapse or immunophenotyping of circulating CD20-positive NHL cells from peripheral blood)
- Current measurable disease, i.e. measurable in two perpendicular dimensions on physical examination or computerized tomography (CT) scan using standardized response criteria for NHL (Cheson et al21, 1999) (see appendix B)
- Age > 18 years
- WHO performance status 0, 1 or 2 (see appendix E)
- Life expectancy of at least 3 months
- Absolute neutrophil count > 1.5 x 10^9/l and platelet count > 100 x 10^9/l (unless caused by NHL infiltration in the bone marrow)
- Written informed consent
Inclusion criteria 90Y-ibritumomab tiuxetan consolidation:
- Patients with PR or CR after 4 cycles of R-PECC
- Less than 25% bone marrow involvement (confirmed by bone marrow biopsy of at least 1.5 cm, if initially positive)
- WHO performance status 0, 1, or 2
- Time interval since the start of the 4th R-PECC between 6 and 12 weeks
- No rituximab-related adverse event necessitating stopping of rituximab administration
- Exclusion Criteria:
Exclusion criteria R-PECC induction
- Prior allogeneic stem cell transplantation
- Prior radioimmunotherapy
- Patients who have received chemotherapy or radiotherapy within 6 weeks prior to study entry or who have not recovered from toxicities related to prior therapies
- Eligibility for ASCT
- ASCT within 12 months of study entry
- Investigational drugs within 4 weeks prior to entry on this study or persistent toxic side effects of such therapy
- Treatment with external-beam radiation therapy to more than 25% of active bone marrow (see appendix F)
- A history of intolerance to rituximab
- Severe cardiac, pulmonary, neurological, psychiatric or metabolic disease which could compromise participation in the study, or serious underlying medical conditions which could impair the ability of the patient to participate in the trial
- Hepatic dysfunction, bilirubin or transaminases ≥ 2.5 x upper normal limit (unless caused by the NHL)
- Renal dysfunction, serum creatinine ≥ 180 µmol/l or clearance ≤ 40 ml/min (unless caused by the NHL)
- Active uncontrolled infections
- Patients known to be HIV-positive
- Current or chronic hepatitis B or hepatitis C infection
- Symptomatic NHL localization in the central nervous system (CNS). Lumbal puncture is not required unless CNS involvement with NHL is clinically suspected
- Transformed indolent lymphoma
- Post-transplant lymphoproliferative disorder
- Pregnant or breast-feeding female patients. Negative serum pregnancy test at study is mandatory for female patients of childbearing potential
Exclusion criteria 90Y-ibritumomab tiuxetan consolidation. All exclusion criteria for R-PECC induction, plus the following:
- Extensive pleural effusion or ascites
- Impaired bone marrow reserve, as indicated by one or more of the following:
- Absolute neutrophil count < 1.5 x 10^9/l, platelet count < 100 x 10^9/l, Hemoglobin < 5.0 mmol/l
- Hypoplastic bone marrow at biopsy
- Administration of systemic corticosteroids at doses higher than 20 mg/day prednisolone or equivalent 2 weeks prior to 90Y-ibritumomab tiuxetan administration.
Registration Details
The patient should be registered immediately after satisfactory completion of screening tests and obtaining informed consent, and before the start of chemotherapy. Patients need to be registered at the HOVON Data Center of the Erasmus MC - Daniel den Hoed via the Internet through TOP (Trial Online Process; https://www.hdc.hovon.nl/top) or by phone call: +31.10.7041560 or fax +31.10.7041028 Monday through Friday, from 09:00 to 17:00. A logon to TOP can be requested at the HOVON website for participants.
The following information will be requested at registration:
- Protocol number
- Institution name
- Name of caller/responsible investigator
- Sex
- Date of birth
- PA number:
- of original diagnosis if refractory disease
- of relapse diagnosis ( if applicable)
- PA laboratory (pathologist and institution) of original or relapse diagnosis
- Eligibility criteria
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
