HOVON HO86 MM
Main info
- Identificatie:
- HOVON 86 MM
- Sponsor:
- HOVON
- Working group party:
- Myeloma
- Age:
- >= 18
- Stadium:
- 2de lijn
- Echelon:
- Level C-HIC&C-SCT
- Included patients:
-
81(of 84)
- Active sites:
-
25(of 35)
- Title:
Phase I/II trial of Lenalidomide plus Bortezomib combined with Dexamethasone in patients in 1st relapse or primary refractory after first line therapy for Multiple Myeloma.
Timeline
News
De HOVON 86 MM studie is op 23JAN12 gesloten. Ik dank u allen hartelijk voor inzet en de eindspurt die er gemaakt is.
De planning is om de resultaten van de eerste 40 patiënten te publiceren op de komende EHA, de resultaten van alle patiënten zal worden ingediend op de komende ASH.
New and updated documents on this website:
*New Insurance certificate for BE sites is available (runs till 31DEC13) (11JUN13) *New WMO insurance certificate for NL sites is available (runs till 01DEC13) (29JAN13) *New WMO insurance certificate for NL sites is available (runs till 01JUL12) (dd 20JUN11)- News letter 5 is available (18MAY11)
- New PPP (Pregnancy Prevention Program is available. please use version 3 instead of version 2)
- CRFs are updated (new version is 09FEB11) due to extraction of patient name code
In relation with the approval of amendment 2, the following documents have been updated on 23DEC10:
*Protocol (dd 11OCT10)- Protocol amendments
- CRF (dd 11OCT10)
- PI&ICF amendment(s)
- CCMO response
- METC approval
- Signature page of protocol
- Registration (&randomisation) form
- Site documents checklist amendment 2
From the CRF set the cytogenetic pages were distracted and a new MM cytogenetic CRF was placed as a separate file under 'other documents' (22JUL10).
There is a new flowchart available that summarises the BM and PB sampling time, called: HO86_flowchart afname materiaal.pdf (added on 02FEB10)
Please find a new version of the WMO insurance certificate for this study below the study documents (added on 28DEC09)
In relation with the approval of amendment 1, the following documents have been updated on 03DEC09:
- Protocol (dd 07OCT09)
- Protocol amendments
- CRF (dd 07OCT09)
- Patient Information & IC form (NL)
- PI & ICF amendment(s)
- CCMO response
- MEC approval
- ABR form
- Signature page of protocol
- Registration (&randomisation) form
- SAE form
- Site documents checklist amendment 1
- Summary of canges ABR form
Previous updated documents:
- Medication Order Form, update June 2009
- Drug accountability form
- Instructions Central Lab, update June 2009
- WMO subject insurance
- HO86 Newsletter 3
In contrast with earlier communications we inform you that compensation of 20% for Velcade costs in this study will not be provided.
For the latest newsletter click under heading 'general' > 'news'. Previous newsletters can be found under 'other documents'
Flow
Details
- Phase:
- Prospective Phase I/II study
- Monitoring Type:
- Not any more
- Objectives:
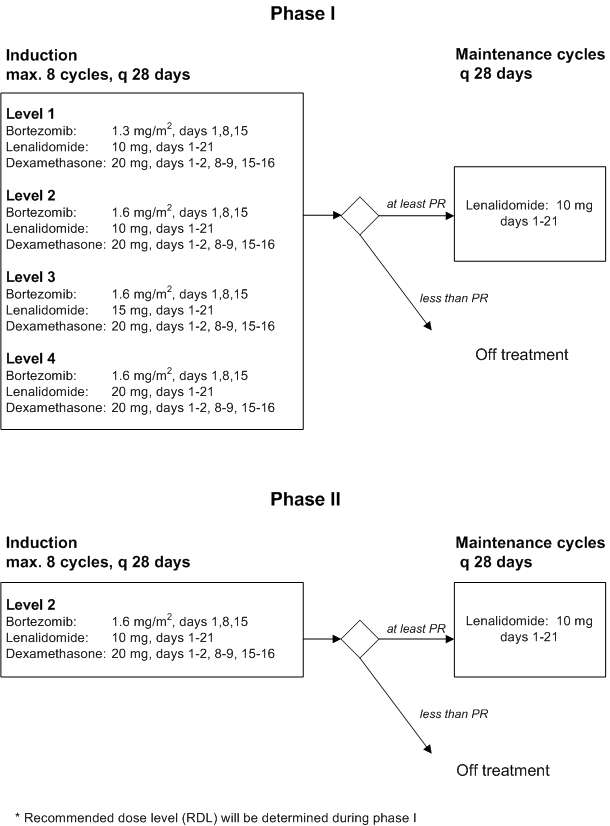
Phase I
Primary objective- To determine the maximum tolerated dose (MTD) and recommended phase II dose level (RDL) of Bortezomib administered once weekly, and of Lenalidomide administered for 3 weeks when combined with Dexamethasone in a 28-days schedule. See paragraph 14 for definitions of MTD and RDL
Secondary objectives
- To evaluate toxicity, especially myelosuppression, polyneuropathy and thrombosis
Phase II
Primary objective- To investigate the efficacy of a maximum of 8 cycles of Bortezomib plus Lenalidomide with Dexamethasone at the RDL, as determined by the (s)CR+VGPR rate
Secondary objectives
- To evaluate the effect of maintenance treatment with Lenalidomide in patients who have achieved any response (sCR, CR, VGPR, or PR) upon treatment with the combination of Bortezomib plus Lenalidomide with Dexamethasone
- To evaluate toxicity, especially myelosuppression, polyneuropathy and thrombosis
- To evaluate progression free survival
- To evaluate overall survival
Eligibility
- Inclusion Criteria:
- Multiple Myeloma Salmon/Durie stage II/III A+B
- Primary refractory to or first relapse after previous objective response (PR, VGPR, CR) on standard first-line treatment
- Not a candidate for high-dose therapy
- Age >= 18 years
- Measurable disease, i.e., serum M-component (>10 g/l), or urinary light-chain excretion (>200mg/24h),or abnormal FLC ratio with involved free light chain (FLC) > 100 mg/l or proven plasmacytoma by biopsy
- Able and/or willing to use adequate contraceptives (especially male patients)
- Written informed consent
- Exclusion Criteria:
- Patient is unable or unwilling to adhere to the requirements of the Lenalidomide Pregnancy Prevention Risk Management Plan.
- Prior therapy with Bortezomib or Lenalidomide
- History of allergic reaction attributable to compounds containing boron or mannitol.
- Peripheral neuropathy or neuropathic pain Grade 2 or higher as defined by NCI CTCAE version 3.
- AL amyloidosis
- Uncontrolled or severe cardiovascular disease:
- New York Heart Association (NYHA) Class III or IV heart failure
- Myocardial infarction within the last 6 months of study entry
- Reduced left ventricular function with an ejection fraction < 50% as measured by MUGA scan or echocardiogram (another method for measuring cardiac function is acceptable)
- Unstable angina
- Unstable cardiac arrhythmias
- Clinically significant pericardial disease
- Impaired hepatic or renal function as defined by:
- ALT and/or AST > 3 x normal value
- Bilirubin > 3 x normal value
- Serum creatinin > 3 x normal value (after adequate hydration)
- Concurrent severe and/or uncontrolled medical condition (e.g. uncontrolled diabetes, infection, hypertension, etc.)
- Known HIV positivity
- History of active malignancy during the past 5 years, except basal carcinoma of the skin or stage 0 cervical carcinoma
Registration Details
The patient should be registered immediately after diagnosis of relapse, and before the start of protocol treatment. Patients need to be registered at the HOVON Data Center of the Erasmus MC Rotterdam – location Daniel via the Internet via TOP (Trial Online Process; https://www.hdc.hovon.nl/top) or by phone call: +31.10.7041568 or fax +31.10.7041028 Monday through Friday, from 09:00 to 17:00 CET. A logon to TOP can be requested at the HOVON Data Center for participants.
The following information will be requested at registration:
- Protocol number
- Institution name
- Name of caller/responsible investigator
- Local patient code (optional)
- Sex
- Date of birth
- Date written informed consent
- Eligibility criteria
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
