HOVON HO902 NHL
Main info
- Identificatie:
- HO902 NHL
- Sponsor:
- HOVON
- Working group party:
- Lymphoma
- Age:
- >= 18
- Stadium:
- 1st lijn
- Echelon:
- Level D
- Included patients:
-
250(of 250)
- Active sites:
-
55(of 62)
- Title:
A national study for blood based response monitoring of newly diagnosed aggressive B-cell lymphoma (DLBCL and HGBCL) patients
Timeline
Flow
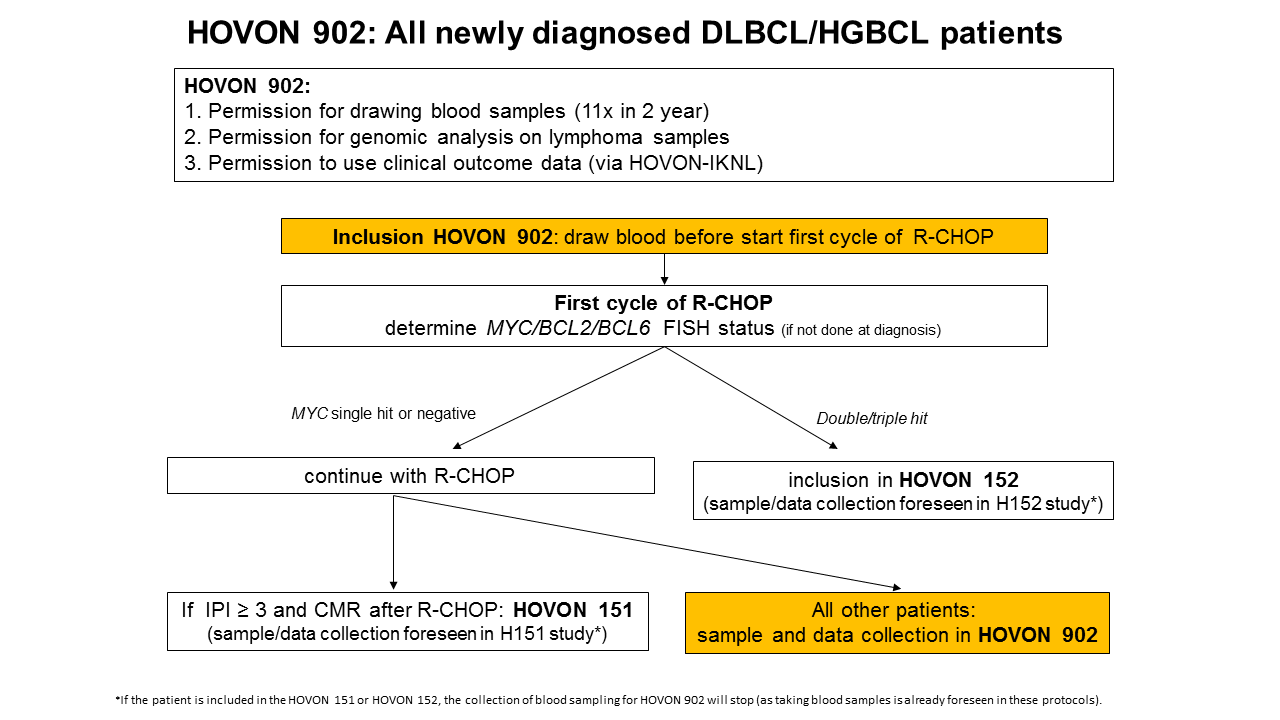
Details
- Phase:
- Observational Prospective
- Monitoring Type:
- Objectives:
The primary objective is to obtain LB samples to develop a reliable blood based assay that identifies primary refractory and early relapsing patients, thus enabling clinical interventional trial in the near future.
The secondary objectives are to collect clinical data and pre-treatment tissue samples to allow for determining molecular profiles and to allow for correlation to the blood based assay.
Eligibility
- Inclusion Criteria:
- Patients diagnosed with newly diagnosed DLBCL (including HGBCL-NOS and HGBCL-DH) according to WHO classification 2016
- Ann Arbor stage II-IV
- Patients intended to be treated with 6 cycles R-CHOP (or DA-EPOCH-R) as first-line treatment (successive inclusion in HOVON 151 or HOVON 152 is possible)
- Age ≥ 18 years
- Exclusion Criteria:
- Patients with limited stage I,II disease planned to receive 3 cycles of R-CHOP + radiotherapy, or 4 x R-CHOP+ 2R
Registration Details
Eligible patients should be registered before start of treatment. Patients need to be registered at the HOVON Data Center by one of the following options:
- HOVON Data Center registration database (www.hovon.nl). Account for registration and a registration manual can be requested at HOVON Data Center.
- By e-mailing the completed registration CRF to hdc@erasmusmc.nl Monday through Friday from 09:00 to 17:00 CET
- By phone +31.10.7041560 Monday through Friday, from 09:00 to 17:00 CET
The following information will be requested at registration:
- Protocol number
- Institution name
- Name of caller/responsible investigator
- Sex
- Year of birth or age at registration
- Date written informed consent
- Specific items for which this patient gives consent (see ICF)
- Date of diagnosis
- Diagnosis (DLBCL or HGBCL)
- Stage II or III or IV
- LDH and ULN
- WHO performance status
- Extranodal localizations 0 or ≥1
- Name of the local pathology laboratory
- Local pathology registration number
- Scheduled date start treatment
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
